Vincenzo Carbone1, Irene Balelli2, Yves Coudière3, Oscar Camara4, Beatriz Trenor5, Michèle Barbier2, Maxime Sermesant2.
1 InSilicoTrials Technologies
2 Inria Center at University Côte d’Azur, Sophia Antipolis, France
3 Université de Bordeaux, L’institut des maladies du rythme cardiaque, France
4 Universitat Pompeu Fabra, Spain
5 Universitat Politècnica de València – Centro de investigacion e innovacion en bioingenieria, Spain
The advantages of in silico approaches based on computational modelling and simulation (CM&S) are clear: they can help reduce development costs, shorten timelines, improve early predictions of safety and efficacy, identify potential failures before reaching human trials and promote personalised solutions. High-resolution biological data, together with advances in computational power and artificial intelligence, are gradually overcoming the complexity of modelling the human body and enabling more accurate predictions.
However, a significant unmet need remains: the lack of accessible, trustworthy, validated in silico models that are robust enough for regulatory adoption. The requirements for technical computational skills and the need for extensive verification, validation and credibility assessment, along with substantial investment in software licences and hardware infrastructure, have considerably limited their adoption among large pharmaceutical and medical device companies, or offered as dedicated consulting services, while remaining an insurmountable challenge for small and medium-sized enterprises.
In silico trials: fostering innovation and resilience to empirical failure
In silico medicine represents a revolutionary approach in the field of medical research and development, and it is at the basis of the current e-healthcare revolution. The use of computer simulations to model biological processes is transforming the design, development, and assessment of new medical products by offering powerful support to traditional experimental methods. By enabling digital testing of medical interventions (devices and drugs), in silico methodologies have the potential to reduce reliance on clinical trials, enhance regulatory assessments and accelerate innovation.
In silico trials can be applied at every stage of a medical product development and life cycle, from preclinical studies, allowing for refining, reducing and potentially replacing benchtop, in vitro and ex vivo experiments as well as in vivo studies on animals, to the clinical context and up to the continuous assessment upon marketing (Viceconti, 2021). Depending on the addressed stage, in silico tools can assist from concept to verification and certification, making predictions about mechanical performance, durability and resistance, as well as deployment effects and interaction with human anatomy (Favre et al., 2021; Miller et al., 2023; Rodero et al., 2023). Also, in silico techniques can provide evidence on toxicity, efficacy, optimal dosing strategies and study design, as well as leverage post-market real-world data (Marshall, 2019). This allows us to investigate several scenarios while minimising interventions on real individuals, which is far more ethically sound than traditional ‘trial and error’ approaches.
In silico trials are gaining visibility and increasing acceptance by regulatory authorities such as the U.S. Food and Drug Administration (2021) and the European Medicines Agency (EMA, 2018), which are gradually integrating them into their evaluation frameworks and including them in the new EU Medical Device Regulation (European Union, 2017). Dedicated organisations have been created, such as the Virtual Physiological Human Institute (vph-institute.org), whose mission is to ensure that the in silico medicine is fully realised, universally adopted and effectively used both in research and clinic; and the Avicenna alliance (avicenna-alliance.com), an association of industry and renowned academia/ healthcare organisations, whose mission is to advocate for the regulation and deployment of in silico methodologies to deliver faster safer and more affordable health care to the patients. However, the widespread adoption and acceptance of in silico trials requires a standardised framework to ensure consistency, reliability and regulatory compliance.
SimCardioTest cloud-based platform
The SimCardioTest project, funded by the European Commission (2021–2025), has successfully realised an easy-to-use in silico trials platform, developed by partner InSilicoTrials Technologies, to host the advanced healthcare simulation solutions developed by other project partners for the virtual testing of cardiac medical devices and drugs, and made them available to medical device and pharmaceutical companies through a cloud-based modelling and simulation as a service (MSaaS) approach (Figure 1).
With the SimCardioTest platform, users can easily set up and launch simulations via any web browser, without the need for computational expertise and dedicated hardware/software infrastructure. Once the simulation is completed, the user can visually process the results and export the simulation outcome in an automatically formatted report. The original models are protected from download or edit, thus preserving the intellectual property of the model creators.
The SimCardioTest platform offers a collaborative space where multiple users with different domain expertise can collaborate on the design and execution of in silico trials from anywhere in the world. The dashboard allows you to monitor the status of ongoing simulations and retrieve the results of all previously run simulations, which are securely stored in a private database. The documentation repository provides a user-friendly manager to simplify and streamline file organisation and management. It supports advanced features such as file updates and versioning for collaborative projects. Components such as access and identity management, front-end and back-end, database, computing scalable cluster, security, data protection and logs are managed through the Microsoft Azure Cloud services, to ensure security and privacy compliance.
Three different use cases (UCs) have been addressed within SimCardioTest, covering several domains in cardiovascular medicine.
Use case 1 – Pacing devices for bradycardia: how does a new lead design affect the capture threshold?
This workflow allows to quantify the electrical properties of cardiac stimulation devices, using two different pipelines built upon the electrophysiology models developed by partners Inria and University of Bordeaux:
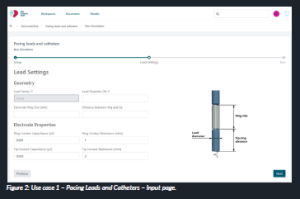
- Energy thresholds, where the user is required to input the geometrical properties of the device and the properties of the bio-electrode contact impedance (Figure 2)
- Population statistics (in silico trial), where the user can add additional information about the stimulation duration and size of the tip scar.
Once the simulation is completed, the user can identify the limit of capture and non-capture domains in diagrams of stimulation amplitude against stimulation duration and outcome statistics over the population of capture/non-capture and delivered energies.
Use case 2 – Left atrial appendage occluders (LAAO): how do device configurations and patient-specific LA/LAA geometries reduce device-related thrombosis?
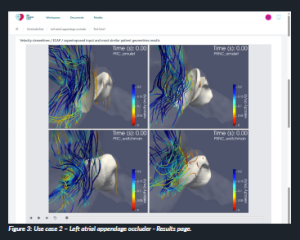
This workflow enables to obtain personalised haemodynamic indices for LA geometries and the identification of the optimal LAAO configuration. The user uploads the geometry file of a new patient’s anatomy and can provide additional inputs describing the patient’s sex, age, weight, height and clinical history. Then, fluid simulation results of the most similar patient are fetched from a virtual dataset of pre-computed cases developed by partner Universitat Pompeu Fabra. The results are visualised through a newly developed 3D viewer (Figure 3), showing the LA anatomy with four different implanted LAAO device configurations: Amplatzer™ Amulet™ covering the pulmonary ridge (PRC_amulet); Amplatzer™ Amulet™ not covering the pulmonary ridge (PRNC_amulet); WATCHMAN™ covering the pulmonary ridge (PRC_ watchman); WATCHMAN™ not covering the pulmonary ridge (PRNC_watchman).
Use case 3 – Preclinical drug development: how do cardiac electrophysiological models inform about drug-induced Torsades de Pointes?
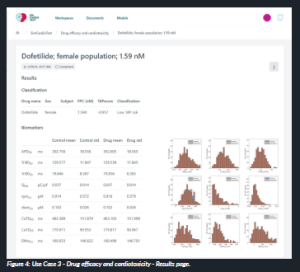
This workflow enables to run in silico trials to test the cardiac safety and efficacy of marketed drugs or new compounds, leveraging electrophysiology and electromechanics cardiac simulations developed by our partner, Universitat Politècnica de València. The user is required to input the concentration value and the ion channel block values of the compound, as well as the characteristics of the virtual population of interest (such as male or female, and healthy or with heart failure history populations). Once the simulation is completed, the results show the effect of the compound on a list of specific biomarkers, assessing the safety of this drug and/or its efficacy under a specific diseased situation (Figure 4).
Added value and benefits for stakeholders in healthcare
The SimCardioTest cloud-based platform provides easy access to computational solutions to speed up and optimise the development process of medical products. The platform operates within a safe and scalable cloud environment, with no on-premises software and hardware requirements, conforming to streamlined processes and following regulatory guidelines.
The platform allows users to simulate different realistic and complex clinical trial designs, evaluating the impact of the selected patient groups and the design characteristics on the performance of the trial.
SimCardioTest platform benefits several key stakeholders:
- Medical devices and pharmaceutical companies
By reducing the time and costs associated with clinical trials, the platform helps industry bring new products to market faster and more efficiently. The virtual patient populations and simulations also provide deeper insights into patient responses to new treatments, helping to identify potential side effects and improving efficacy. - Clinical research organisations
SimCardioTest offers access to virtual patient populations and simulations that enable the development and evaluation of new treatments. This accelerates the development process, allowing researchers to identify new treatments for diseases and conditions more efficiently. - Regulators
By providing more accurate and efficient clinical trial data, SimCardioTest can help regulators make informed decisions about the safety and efficacy of new drugs. This can lead to faster approvals and better outcomes for patients.
- Patients
By reducing the need for large-scale, in-person clinical trials, SimCardioTest helps to reduce the risk of harm to patients and makes it easier for more patients to participate in trials. This is particularly important for patients with rare diseases or conditions, who may have difficulty finding trials to participate in.
As a pioneering innovator in the field of clinical trials and healthcare technology in Europe, partner InSilicoTrials Technologies has been awarded the prestigious 2023 Innovation Radar Prize in the AI and Smart Devices category by the European Commission: “For their EU-funded computational modelling and simulation platform based on digital twins and biosimulation to optimise clinical trials.”
Conclusion
The SimCardioTest cloud-based platform offers healthcare companies and researchers integrated and user-friendly simulation workflows for in silico testing during the development and validation of new cardiac medical devices and drugs. Based on a web-based collaboration paradigm, the platform represents an innovative solution for the life sciences and healthcare environments, ultimately resulting in an increased pace of innovation and a reduced time-to-market for new treatments.
Project summary
Cardiovascular diseases affect 15 million people in Europe, and digital solutions are now seen as very useful tools in the search for new drugs and medical devices. SimCardioTest is a four-year project funded by the European Commission that aims to develop credible computer modelling and simulation approaches on a cloud-based platform for testing cardiac drugs and devices in silico.
Project lead
SimCardioTest is led by Inria, the French national research institute for the digital sciences. A world-class research and technological innovation organisation, Inria develop and support scientific and entrepreneurial projects that create value in France and Europe. Dr Maxime Sermesant, Head of Computational Cardiology at Inria Epione and Chair of AI and Biophysics at 3IA Côte d’Azur, ensures the scientific co-ordination.
Project partners
SimCardioTest brings together leading experts in the field of cardiac simulation, drug effect, medical devices and regulatory process. It includes a large company (Microport – CRM), SMEs (InSilicoTrials Technologies), research organisations (Inria and Simula), universities (University of Bordeaux, University Pompeu Fabra, Polytechnic University of Valencia), and an international non-profit organisation (The Virtual Physiological Human Institute).
CONTACT DETAILS
Michèle Barbier
Inria Sophia Antipolis-Méditerranée,
2004 Route des Lucioles, 06902 Valbonne,
France
Email: michele.barbier@inria.fr
Web: https://www.simcardiotest.eu
X: @SimCardioTest
LinkedIn: SimCardioTest
Funding disclaimer
This project has been funded by European Union’s Horizon 2020 research and innovation programme – grant agreement number 101016496.
Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the granting authority can be held responsible for them.
References
European Medicines Agency (EMA) (2018) Work plan for the Modelling and Simulation Working Group (MSWG) for 2018. Amsterdam: European Medicines Agency.
European Union (2017) Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. Official Journal of the European Union, L117, 5 May, pp. 1–175.
Favre, P., Maquer, G., Henderson, A., Hertig, D., Ciric, D. and Bischoff, J.E. (2021) ‘In silico clinical trials in the orthopaedic device industry: from fantasy to reality?’, Annals of Biomedical Engineering, 49, pp. 3213–3226. doi: 10.1007/s10439-021-02787-y.
Marshall, S., Madabushi, R., Manolis, E., Krudys, K., Staab, A., Dykstra, K. and Visser, S.A.G. (2019) ‘Model-informed drug discovery and development: current industry good practice and regulatory expectations and future perspectives’, CPT: Pharmacometrics & Systems Pharmacology, 8, pp. 87–96. doi: 10.1002/psp4.12372.
Miller, C., Konduri, P., Bridio, S., Luraghi, G., Terreros, N.A., Boodt, N. and Hoekstra, A. (2023) ‘In silico thrombectomy trials for acute ischemic stroke’, Computer Methods and Programs in Biomedicine, 228, p. 107244. doi: 10.1016/j.cmpb.2022.107244.
Rodero, C., Baptiste, T.M.G., Barrows, R.K., Keramati, H., Sillett, C.P., Strocchi, M., Lamata, P. and Niederer, S.A. (2023) ‘A systematic review of cardiac insilico clinical trials’, Progress in Biomedical Engineering, 5(3), article 032004. doi: 10.1088/25161091/acdc71.
U.S. Food and Drug Administration (FDA) (2021) Advancing regulatory science at FDA: focus areas of regulatory science (FARS).
Viceconti, M., Emili, L., Afshari, P., Courcelles, E., Curreli, C., Famaey, N., Geris, L., Horner, M., Jori, M.C., Kulesza, A., Loewe, A., Neidlin, M., Reiterer, M., Rousseau, C.F., Russo, G., Sonntag, S.J., Voisin, E.M. and Pappalardo, F. (2021) ‘Possible contexts of use for in silico trials methodologies: a consensusbased review’, IEEE Journal of Biomedical and Health Informatics, 25(10), pp. 3977–3982. doi: 10.1109/JBHI.2021.3090469.
Figure legends
Figure 1: Home page of SimCardioTest cloud-based platform.
Figure 2: Use case 1 – Pacing Leads and Catheters – Input page.
Figure 3: Use case 2 – Left atrial appendage occluder – Results page.
Figure 4: Use Case 3 – Drug efficacy and cardiotoxicity – Results page.