The RECGLYCANMR project is breaking new ground in glycan research. The project team is unravelling how glycans interact in our bodies by using advanced techniques like nuclear magnetic resonance (NMR). Their findings hold promise for developing effective treatments for various diseases.
Glycans are everywhere. Every single cell, from the simplest bacteria to complex organisms, as human beings, is covered by a layer of glycans. They influence a myriad of biological events of biomedical importance. Therefore, these molecules hold tremendous potential in the quest for new therapeutics.
RECGLYCANMR employs nuclear magnetic resonance (NMR) as a key tool for studying, at atomic resolution, molecular recognition processes in which glycans are involved. Till now, sugar recognition by NMR was limited to in vitro protocols. However, the exquisite glycan selectivity of lectins is strongly influenced by the environment. Therefore, RECGLYCANMR studies these interactions in the biological context, with a multidisciplinary approach of state-of-the-art chemical biology, in-cell structural chemistry, and biophysics to answer pivotal questions related to sugar recognition in Nature and their implications for finding solutions for diseases. Thus, the synergic employment of methods to detect binding with atomic resolution (NMR, X-Ray), to characterise the dynamic features of the players (NMR, MD) and to deduce binding affinities (BLI, ITC) is opening new horizons in the field.
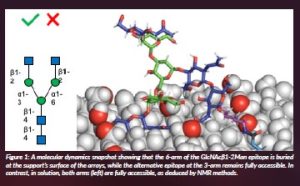
Understanding the role of presentation and multivalency on glycan recognition in Nature is one key issue within RECGLYCANMR (Quintana et al., 2023). Indeed, the presentation of glycans on biological membranes and their intrinsic chemical characteristics display a major impact on recognition. From the methodology perspective, the interaction features of a key lectin of the human immune system, LSECtin, toward a variety of asymmetric N-glycans have been disentangled in solution by NMR and compared to those taking place under surface conditions, using glycan microarrays (Bertuzzi et al., 2022). In particular, the employment of a couple of glycoisomers (LDN6, LDN3) and their parent non-elongated N-glycan (G0, Figure 1) were employed to unravel the specific binding modes that occur under both experimental set-ups. Strikingly, opposite to the observations deduced in microarrays, where only LDN6 was bound by L-sectin, both LDN3 and LDN6 asymmetric N-glycans interact with the lectin in a fairly similar manner in solution. In conclusion, it is evident that drastically divergent results can be obtained using diverse experimental methods, highlighting the tremendous jump that exists when translating results obtained in vitro to the in vivo environment.
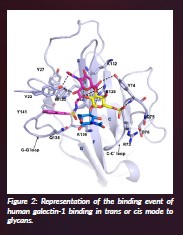
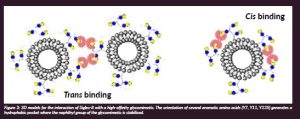
Glycans are usually presented in a multivalent manner on the cell surface in the glycocalyx. Indeed, as described in the preceding paragraph, the presentation of glycans may drastically impact their binding by lectins, highly affecting the corresponding binding affinity and even selectivity. In this context, we have studied the interaction of a variety of multivalent lactose-functionalised glycomacromolecules and their lipid-conjugates with two human galectins. We have employed as ligands liposomes decorated with those glycomacromolecules to evaluate their interactions in a cell-mimicking environment (Lete et al., 2023). Key details of the interaction have been unravelled by NMR complemented by cryo-EM methods and molecular dynamics. It was deduced that hGal-1 dimers crosslink the lactose moieties in the glycomacromolecules, with the interactions taking place in cis, within the same liposome (Figure 2), or in trans, connecting disaccharides at different liposomes, evidencing the key role of presentation of the lectin and the glycan to provide efficient binding (Quintana et al., 2023).
Human sialic acid-binding immunoglobulin-like lectin receptors are intimately linked to the human immune response. We have focused (Lenza et al., 2022) on Siglec-8, an inhibitory receptor that inhibits mast cell degranulation when bound by sialylated glycans or specific monoclonal antibodies (mAbs). Indeed, Siglec-8 is a key negative regulator of inflammatory responses in several diseases, including allergic airway inflammation. We have deciphered the recognition features of the interaction between Siglec-8 and lirentelimab (2C4), a monoclonal antibody under clinical development. Moreover, we have also unravelled its interaction with a sialoside mimetic, both with the potential to suppress mast cell degranulation. The analysis of the 3D structure of Siglec-8 bound to the fragment antigen-binding portion of the 2C4 mAb was solved by X-ray crystallography. Fittingly, the 2C4 antibody interacts with Siglec-8 very close to its sialic acid recognition domain. The interaction of Siglec-8 with a small molecule, a high-affinity glycomimetic of its natural sialic acid ligand (9-N-napthylsufonimide-Neu5Ac, NSANeuAc), was unravelled using a synergetic combination of X-ray and NMR. It was shown that the sialoside ring of NSANeuAc binds to the canonical sialyl binding pocket, always present in the Siglec family. These investigations have provided novel clues for advancing the rational design of the next generation of Siglec-8 inhibitors.
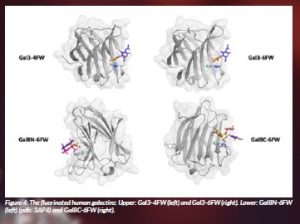
One key family of glycan receptors in Nature are galectins, which display a myriad of biological functions related to disease. In RECGLYCANMR, we are exploring the interaction of galectins with glycans, especially the histo blood group antigens, since it has been shown that hGal-4 binds and destroys bacteria that express the blood group B antigen. In this context, we have investigated the incorporation of 19F-NMR labels into galectins to exploit the features of the fluorine nucleus to monitor binding to glycans by using NMR (Lete et al. 2023). In particular, human Galectin-3 (hGal-3) and human Galectin-8 (hGal-8) with one and two sugar recognition domains, respectively, have been employed. While h-Gal3 displays one Trp in its amino acid sequence, hGal-8 shows three of them, one at each sugar-binding site and a third one that is not involved in binding to glycans. All of them were replaced by F-Trp rings. Fittingly, the presence of the fluorine atom did not change the binding affinity towards the ligands. From a technical perspective, the binding events were in slow exchange on the 19F NMR chemical-shift timescale and allowed us to independently observe and monitor the binding events that were taking place at the two different binding sites within h-Gal8. This approach provides novel avenues to investigate glycan-galectin interactions involved in health and disease.
References
Bertuzzi, S., Peccati, F., Serna, S., Artschwager, R., Notova, S., Thépaut, M., Jiménez-Oses, G., Fieschi, F., Reichardt, N. C., Jiménez-Barbero, J. and Arda, A. (2022) ‘Immobilization of biantennary N-glycans leads to branch specific epitope recognition by LSECtin’, ACS Central Science, 8(10), pp. 1415–1423. doi: 10.1021/acscentsci.2c00719.
Lenza, M. P., Atxabal, U., Nycholat, C., Oyenarte, I., Franconetti, A., Quintana, J. I., Delgado, S. Núñez-Franco, R., Garnica Marroquín, C. T., Coelho, H. Unione, L., Jiménez-Oses, G., Marcelo, F., Schubert, M., Paulson, J. C., Jiménez-Barbero, J. and Ereño-Orbea, J. (2022) ‘Structures of the Inhibitory Receptor Siglec-8 in Complex with a High-Affinity Sialoside Analogue and a Therapeutic Antibody’, JACS Au, 3(1), pp. 204–215. doi: 10.1021/jacsau.2c00592.
Lete, M. G., Franconetti, A., Bertuzzi, S., Delgado, S., Azkargorta, M., Elortza, F., Millet, O., Jiménez-Osés, G., Arda, A. and Jiménez-Barbero, J. (2023) ‘NMR Investigation of Protein-Carbohydrate Interactions: The Recognition of Glycans by Galectins Engineered with Fluorotryptophan Residues’, Chemistry: A European Journal, 29(5), e202202208. doi: 10.1002/chem.202202208.
Lete, M. G., Hoffmann, M., Schomann, N., Martinez-Castillo, A., Peccati, F., Konietzny, P., Delgado, S., Snyder, N., Jiménez-Osés, G., Abrescia, N., Ardá, A. Hartmann, L. and Jiménez-Barbero, J. (2023) ‘Molecular Recognition of Glycan-Bearing Glycomacromolecules Presented at Membrane Surfaces by Lectins: An NMR View’, ACS Omega, Just accepted.
Quintana, J. I., Atxabal U., Unione L., Ardá A. and Jiménez-Barbero, J. (2023) Exploring multivalent carbohydrate-protein interactions by NMR. Chemical Society Reviews, 52(5), pp. 1591–1613. doi: 10.1039/d2cs00983h.
Project name
RECGLYCANMR
Project summary
Breaking the limits in glycan recognition by NMR (RECGLYCANMR) is a multidisciplinary project that combines state-of-the-art chemistry and chemical biology methods, including in vitro and in-cell NMR, and biophysics protocols under crowding conditions to ask and answer pivotal questions related to sugar molecular recognition in Nature that are intimately related to infection and inflammation diseases.
Project partners
RECGLYCANMR is based at the Center for Cooperative Research in Biosciences (CIC bioGUNE, Bilbao), taking advantage of the state-of-the-art NMR facility and technology platforms (crystallography, electron microscopy, biophysics, computing cluster, oligosaccharide solid-phase synthesis) at this multidisciplinary centre.
Project lead profile
Jesús Jiménez-Barbero has been Ikerbasque Professor and Scientific Director of CIC bioGUNE since 2014. He received his PhD in Madrid (1987). Earlier in his career, he worked at Grenoble, Zurich, Mill Hill and Pittsburgh. His investigations on glycan-receptor interactions have been disseminated through more than 600 scientific publications and more than 250 invited and plenary lectures.
Project contacts
Bizkaia Technology Park, Building 801A; 48160 Derio, Spain
+34944061300
Jesús Jiménez-Barbero
Itziar Gil de la Pisa
Funding
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 788143.