Targeted drug delivery is one of the most essential objectives in cancer treatment due to the high toxicity of chemotherapy drugs. Great effort has been invested in delivering drugs specifically to the site of disease, i.e. the tumour area, while reducing the exposure of healthy tissues. To achieve this specificity, there are many attempts to produce the ‘active targeting’ of drug delivery systems. Nano or microparticles may be loaded with active drug molecules and function as drug carriers. An optimal design would enable the drug carrier to recognise the tumour cells and attach to them while ‘ignoring’ normal cells. To realise such a design, it is essential to identify the unique features of the tumour cells that can potentially be exploited to guide the drug to act specifically on the tumour with minimal harm to healthy tissues.
The traditional approach of active drug delivery focuses on decorating drug carriers with molecules that can recognise unique components on the surface of cancer cells. However, a major problem in this approach is that cancer cells’ surface molecules constantly change during the course of the disease, thus making the targeting less efficient over time.
A new principal for cell selectivity
An innovative project, MTrix, suggests a new and ‘out of the box’ solution to this problem. Prof. Benny’s laboratory from The Hebrew University of Jerusalem found a new way to eliminate the dependency of drug targeting on cell surface molecules and instead use cells’ physical properties as a principle for cell selectivity.
There is growing evidence correlating cancer aggressiveness with the mechanical deformability of tumour cells. Cancer cells have an enhanced ability to change their shape in response to stimuli compared with normal cells derived from the same tissue origin. Various processes of cancer development require mechanical adjustments of the tumour cells to physical environments. For instance, the growth and development of high-pressure tumours, the detachment of metastases-forming-cells from the primary tumour, their efficient motion and passage through narrow confinements, and their establishment in different locations (Chen et al., 2016; Baker et al., 2010; Wirtz, Konstantopoulos and Searson, 2011; Friedl and Wolf, 2003; Butcher, Alliston and Weaver, 2009).
The mechanical properties of cells can be measured using biophysical methods, usually by detecting the response of cells to applied force (Gavara, 2017; Brill-Karniely, 2020). For many cancers, the higher the tendency of the tumours to metastasise, the more the cells are elastic and deformable (Brill-Karniely et al., 2020; Suresh, 2007).
Considering the major role of cell elasticity in cancer cell function and the variance in the ability of cancer versus normal cells to deform, the question is whether these can be utilised to design specific nanocarriers that can penetrate distinctively into cancer cells, thus providing a desired ‘active drug targeting’. In other words, the physical differences between normal and cancer cells may be taken advantage of to promote drug delivery specificity.
A triangular correlation
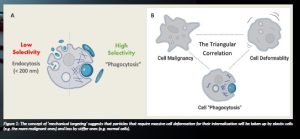
As per physical theories, cell deformability is essential for inserting particles, e.g. drug delivery systems, into cells (Brill-Karniely et al., 2020; Stern et al., 2016). The more the cells are elastic, the higher their tendency to absorb particles that can further internalise into the cells. The theoretical prediction of the researchers was that by changing the physical properties of nano and microparticles, such as the size, shape or elasticity, the specificity of drug delivery to cancer is largely enhanced, as demonstrated in Figure 1A. Indeed, in a detailed study of melanoma vs normal skin cells, the researchers found that cancer cells have a high capacity to internalise nano/microparticles. They demonstrated in various biological platforms, including 2D, 3D and animal models, that there is a ‘triangular correlation’ between cell malignancy, cell deformability and cell ‘phagocytosis’ capacity (Figure 1B) (Brill- Karniely et al., 2020).
The triangular correlation was proven in human prostate cancer cells and human melanoma cancer cells of different malignancy potentials sorted into subpopulations based solely on their particle uptake capacity. The highly phagocytic cells showed elevated aggressiveness ex vivo and in vivo. Most interestingly, the capacity of cells to uptake particles was found to be higher with generational progression, suggesting that there is genetic involvement; indeed, changes in the cell epigenetic signature were found (Brill-Karniely et al., 2020). In all cases, enhanced ‘phagocytosis’ and aggressiveness phenotypes were correlated with greater cell deformability, as predicted by a computational model. The multidisciplinary study provides a novel unconventional approach and a proof of concept that phagocytic measurements can be applied for cancer diagnostics and personalised nanomedicine, in which drug delivery systems can be optimised per person to obtain maximum selectivity.
Future applications
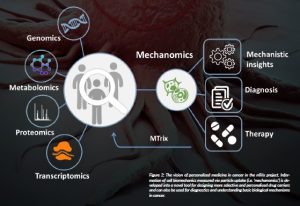
Based on the novel concept, the research opens up the use of ‘mechanomics’, e.g. cell biomechanics data, as a novel channel of information that can be translated into the future diagnostic and treatment of cancer, as illustrated in Figure 2.
References
Baker, E.L., Lu, J., Yu, D., Bonnecaze, R.T. and Zaman, M.H. (2010) ‘Cancer cell stiffness: integrated roles of three-dimensional matrix stiffness and transforming potential’, Biophysical Journal, 99(7), pp. 2048– 2057: doi: 10.1016/j.bpj.2010.07.051.
Brill-Karniely, Y. (2020) ‘Mechanical Measurements of Cells Using AFM: 3D or 2D Physics?’, Frontiers in Bioengineering and Biotechnology, 8:605153. doi: 10.3389/fbioe.2020.605153.
Brill-Karniely, Y., Dror, D., Duanis-Assaf, T., Goldstein, Y., Schwob, O., Millo, T., Orehov, N., Stern, T., Jaber, M., Loyfer, N., Vosk-Artzi, M., Benyamini, H., Bielenberg, D., Kaplan, T., Buganim, Y., Reches, M. and Benny, O. (2020) ‘Triangular correlation (TrC) between cancer aggressiveness, cell uptake capability, and cell deformability’, Science Advances, 6:eaax2861. doi: 10.1126/sciadv.aax2861.
Butcher, D.T., Alliston, T. and Weaver, V.M. (2009) ‘A tense situation: forcing tumour progression’, Nature Reviews Cancer, 9, pp. 108–122. doi: 10.1038/nrc2544.
Chen, J., Zhou, W., Jia, Q., Chen, J., Zhang, S., Yao, W., Wei, F., Zhang, Y., Yang, F., Huang, W., Zhang, H., Zhang, Y., Zhihong, Z., Jia, H. and Wang, N. (2016) ‘Efficient extravasation of tumour-repopulating cells depends on cell deformability’, Scientific Reports, 6, 19304. doi: 10.1038/srep19304.
Friedl, P. and Wolf, K. (2003) ‘Tumour-cell invasion and migration: diversity and escape mechanisms’, Nature Reviews Cancer, 3, pp. 362–374. doi: 10.1038/nrc1075.
Gavara, N. (2017) ‘A beginner’s guide to atomic force microscopy probing for cell mechanics’, Microscopy Research and Technique, 80(1), pp. 75–84. doi: 10.1002/jemt.22776.
Shoval, H., Karsch-Bluman, A., Brill-Karniely, Y., Stern, T., Zamir, G., Hubert, A. and Benny, O. (2017) ‘Tumor cells and their crosstalk with endothelial cells in 3D spheroids’, Scientific Reports, 7, 10428. doi: 10.1038/s41598-017-10699-y.
Stern, T., Kaner, I., Laser Zer, N., Shoval, H., Dror, D., Manevitch, Z., Chai, L., Brill-Karniely, Y. and Benny, O. (2016) ‘Rigidity of polymer micelles affects interactions with tumor cells’, Journal of Controlled Release, pii: S0168-3659(16)30783-0.
Suresh, S. (2007) ‘Biomechanics and biophysics of cancer cells’, Acta Biomater, 3, pp. 413–438. doi: 10.1016/j.actbio.2007.04.002.
Wirtz, D., Konstantopoulos, K. and Searson, P.C. (2011) ‘The physics of cancer: the role of physical interactions and mechanical forces in metastasis’, Nature Reviews Cancer, 11, pp. 512–522, doi: 10.1038/ nrc3080.
PROJECT SUMMARY
The project goal is to provide an integrative approach for the rational and personalised design of drug carriers in cancer, using the mechanical properties of cells and particles as the main principle for drug targeting— based on cell uptake. Our unique approach uses physical differences between normal and cancer cells that can define the capacity of a given particle to be engulfed by a deformable cell in order to promote specificity of therapy. In the project, we aim to establish a correlation between cancer cell deformability, malignancy and ‘phagocytosis’. The project includes advanced technologies of microfluidics, nanotechnology and theoretical physical model.
PROJECT LEAD PROFILE
Prof. Ofra Benny is head of The Lab for Nanomedicine and the Tumor- Microenvironment at the Hebrew University of Jerusalem. Her research focuses on cancer and drug development based on synthetic and natural compounds. Her multidisciplinary approach includes the development of novel nanomedicine tools for drug delivery specific for cancer and key components of the tumour microenvironment. Prof. Benny completed her postdoc in 2007 at the Boston Children’s Hospital and The Vascular Biology programme in Harvard Medical School. She has published several papers in publications such as Nature Biotech and Science Advances; she owns many patents and awards in the field of nanomedicine. Project contacts
Prof Ofra Benny
Institute for Drug Research, The School of Pharmacy, Faculty of Medicine, The Hebrew University of Jerusalem
972-2-6757268
ofra.benny@mail.huji.ac.il
www.benny-lab.com
FUNDING
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 756762.
Figure legends
Figure 1: The concept of ‘mechanical targeting’ suggests that particles that require massive cell deformation for their internalisation will be taken up by elastic cells (e.g. the more malignant ones) and less by stiffer ones (e.g. normal cells).
Figure 2: The vision of personalised medicine in cancer in the MTrix project. Information of cell biomechanics measured via particle uptake (i.e. ‘mechanomics’) is developed into a novel tool for designing more selective and personalised drug carriers and can also be used for diagnostics and understanding basic biological mechanisms in cancer.