Raphaëlle Lesagea,
Roberta De Michelea,
Martina Contina
Oscar Camarab and
Liesbet Gerisa
a Virtual Physiological Human Institute
(VPHi), Belgium
b Universitat Pompeu Fabra,
Barcelona, Spain
The rise of digital tools in healthcare
The healthcare sector is currently experiencing a digital transformation at every level, and cardiovascular medicine is no exception. With the increasing use of digital methods to study diseases, treat patients and create new therapies, the field of in silico medicine, or the use of computer-based tools to predict biomedical and clinical outcomes, has emerged. The term in silico refers to the silica in our computers’ chips.
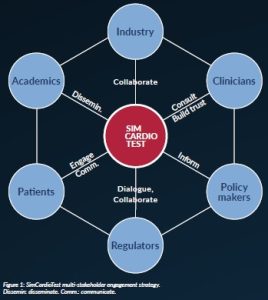
Those in silico methods are highly innovative. Scientists agree on their potential to solve numerous challenges facing cardiovascular therapies, such as the personalisation of treatments, reduction of R&D cost, the inclusion of under-represented sub-populations in clinical trials and regulatory complexity. Nevertheless, they can also disrupt traditional practices. Therefore, it is of utmost importance to involve all relevant stakeholders in the process of their development, including patients, medical practitioners, regulators etc. (Figure 1).
How can in silico approaches help with cardiac health?
In silico technologies are being increasingly used at every step of the cardiac therapy life-cycle. Indeed, there are numerous applications from the stage of medical product design and development to regulatory approval (FDA, 2021a), but also in the clinical practice (Lesage et al., 2022) and for improving patient experience.
SimCardioTest use cases
The SimCardioTest is a Research and Innovation Action funded by the European Commission (2020–2024) to develop new digital tools for cardiovascular therapies. It focuses on developing a cloud-based platform for the virtual testing of cardiac medical devices and drugs. The aim is to lower the need for animal models, increase the number of tested scenarios or refine later phases of trials, etc. Computational models are built and validated for the platform for device manufacturers and pharmaceutical companies to run virtual clinical trials and test parameters such as the long-term mechanical resistance of devices and the safety and efficacy of therapies, among others. Examples of applications covering some of the most relevant heart problems are developed with three use cases:
- Pacing devices used in cardiac arrhythmias associated with
heart failure - Left atrial appendage occluders
used to reduce the risk of stroke - Drugs evaluated to ensure safety, efficacy and limit cardiotoxicity.
For more details, see our article: What computational sciences can do for your heart (Barbier et al., 2021).
Multiple stakeholder engagement
Involving the public and patients
The primary objective of computational medicine is to improve patients’ health and safety. Hence, it is crucial that members of the public and patients are involved in the technology development process. A common misconception by scientists is to see patient outreach and engagement solely as a way to communicate their research to a large audience in layman’s terms towards the end of the research and development phase. Yet, informing the public is a valuable task, strongly encouraged by the European Commission, which emphasises the importance of responsible research and innovation (ERA Learn, no date) as a way to engage and involve various stakeholders to steer innovation in a direction that is meaningful and useful for the society at large.
Patient organisations, such as the European Patient Forum, or more specific ones, such as SAFE (Stroke Alliance for Europe) and the Global Heart Hub, raise the voice of patients (with cardiovascular diseases) and open the dialogue on the digital transformation in healthcare (European Patients’ Form, no date). This underlines the importance of not letting technology development and patient dialogue happen in separate ways.
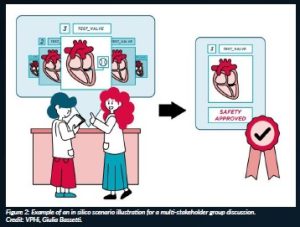
Following that strategy, SimCardioTest organises discussion groups involving patients and medical practitioners about their expectations, needs and concerns related to the in silico technologies being developed in the project. During these discussion groups, amongst others, fictitious scenarios describing the context in which the technology would be used are explained and illustrated to trigger discussions (Figure 2).
The resulting discussions raise awareness about the developed technologies and are fed back to researchers for future guidance. Additionally, they may provide useful recommendations to inform policymakers when appropriate.
Guidelines and standards exist to assist scientists with engaging patients in the development of health technologies. However, there is currently a lack of guidance for in silico technologies in particular. The engagement tools and material produced as part of the SimCardioTest engagement activities are also intended to benefit the broader computational modelling community.
Raising awareness and building trust among medical practitioners
The use of in silico technologies in clinical practice is envisioned and can be used for understanding cardiac pathophysiology, planning interventions, informing the patient, improving diagnosis, or as a collaborative tool for true patient-centric and team-based care (Dassault Systèmes, no date). One point on which most stakeholders agree is that opinions vary between different medical practitioners and countries. The level of familiarity with computational technologies is sparse, and trust or distrust in the technology is not always rightly placed.
However, medical practitioners are often the end-users of the in silico tools or the prescriber of the medical products whose development was based on modelling and simulations. Hence, the necessity of growing visibility, raising awareness and building trust among that community.
This endeavour may take various forms and go through assessing actual situations in the field, consulting, involving clinicians in technological co-creation processes, as well as training, and disseminating relevant information and success stories.
For example, SimCardioTest invites cardiovascular interventionists, surgeons and cardiologists to the discussion groups for patients (see ‘Involving the public and patients’). The aim is to evaluate their expectations and perceived challenges and concerns with respect to the project’s use cases. The reporting of this information will guide the researchers and help inform policymakers as well as the broader in silico medicine community.
On a larger scale, the VPHi and its partners conduct surveys targeting the clinical community to generate actionable public data about the current perception and use of computer modelling and simulation in clinical practice and identify potential barriers and opportunities.
It is important to note that full uptake of in silico technologies for cardiovascular therapy development may also imply targeting other types of clinical actors. Indeed, clinical opinion leaders and trial experts in regulatory bodies also strongly impact that matter.
Dialogue and collaboration with regulators
Opening the dialogue with health regulators is relevant when it comes to getting computer-based tools approved for medical use. Tools based on predictive modelling and the simulations themselves should be approved as software as a medical device (SaMD) for use in medical practice. Besides that, in silico methods employed for supporting the safety and efficacy of medical products in market approval dossiers provide what is commonly called ‘digital evidence’. So, an in silico tool may itself be ‘qualified’ as a methodology for medical product development regulatory agencies such as EMA or FDA.
In practice, things are not straightforward. Many recent digital innovations, such as in silico technologies, do not fit neatly into the traditional market landscape as recognised by existing regulatory bodies. There is a need for knowledge-sharing and collaboration between frontier innovators and regulators to establish how those in silico tools can be validated and accepted as part of regulatory submissions. This is key to achieving the potential of in silico approaches while maintaining high patient-safety standards.
Regulatory stakeholders are currently collaborating with academia and industry to set guidelines and standards on which everyone could rely. For example, regulatory ‘sandboxes’ are being proposed to regulate AI (European Commission, 2023a), and regulators are calling for public feedback on draft guidances for modelling and simulation in healthcare (FDA, 2021b). International guidance may also arise from expanding and harmonising existing standards (e.g. US ASME-V&V40). In addition, ongoing community efforts are establishing good simulation practice (Viceconti, 2021) for quality insurance.
Because use cases and success stories are key to fuelling that dialogue, the vocation of the SimCardioTest use cases is also to provide proof of principle, exemplifying how computer-based technologies may be used and validated.
Informing policymakers
European policy follows its own agenda by prioritising specific thematics and revising pieces of legislation relevant to current affairs. That agenda is regularly disclosed, and policymakers often open calls for public reviews on new draft policies. That is because while policymakers are busy defining the strategy for the future of Europe, they need inputs from field experts and stakeholders to evaluate needs and receive feedback.
The health and digital transformation policy agenda is extremely active given the current context, with the rise of digital tools and AI in all sectors, the era of data—175 zetabytes in 2025 (European Commission, 2022a)—and the world COVID-19 pandemic. For example, the landscape is marked by the recent developments of the EU pharmaceuticals strategy, the European Data (European Commission, 2022b) and AI (European Commission, 2023b) Acts, among other relevant documents.
Consortia, such as SimCardioTest, can group and amplify the voices of individual researchers or stakeholders who have less weight in these matters otherwise. This duty is taken seriously by formulating needs, providing feedback during public calls, and briefing policymakers on the outcome of the stakeholder consultations. This is partly done via the Virtual Physiological Human Institute (VPHi, the international scientific society for in silico medicine) and the Avicenna Alliance (the collaboration of VPHi and industry), reflecting the viewpoints of an even larger part of the in silico community.
Conclusion
The healthcare sector is facing an era of digital transformation in which computational tools are taking an increasingly important role. The cardiovascular field is on the centre stage of that change. Due to the innovative and disruptive nature of those technologies, all stakeholders of the society are concerned with one aspect or another of that change. While developing new technologies, the SimCardioTest consortium is making a point to engage all relevant stakeholders in tailored ways to involve, raise awareness and eventually realise the potential of in silico approaches in cardiovascular health product development.
Project summary
Cardiovascular diseases affect 15 million people in Europe, and digital solutions are now seen as very useful tools in the search for new drugs and medical devices.
SimCardioTest is a four-year project funded by the European Commission that aims to develop credible computer modelling and simulation approaches on a cloud-based platform for testing cardiac drugs and devices in silico.
Project partners
SimCardioTest brings together leading experts in the field of cardiac simulation, drug effect, medical devices and regulatory process. It includes large companies (Microport – CRM), SMEs (ExactCure and InSilicoTrials), research organisations (Inria and Simula), universities (University of Bordeaux, University Pompeu Fabra, Polytechnic University of Valencia) and an international non-profit organisation (The Virtual Physiological Human Institute).
Project lead profile
SimCardioTest is led by Inria, the French national research institute for the digital sciences. A world-class research and technological innovation organisation, Inria develop and support scientific and entrepreneurial projects that create value in France and Europe. Dr Maxime Sermesant, Head of Computational Cardiology at Inria Epione and Chair of AI and Biophysics at 3IA Côte d’Azur, ensures the scientific coordination.
Project partner
Michèle Barbier
Inria Sophia Antipolis-Méditerranée,
2004 Route des Lucioles,
06902 Valbonne, France
+336 3307 9899
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101016.