Anna S. Jung1,2, Alba Mena Gómez1,2 & Alexander Bartelt1,2
1 Chair of Translational Nutritional Medicine, Department of Molecular Life Sciences, TUM School of Life Sciences, Technical University of Munich, 85354 Freising, Germany
2 Else Kröner Fresenius Centre for Nutritional Medicine, Technical University of Munich, 85354 Munich, Germany
Obesity and type 2 diabetes: Europe’s most challenging health problem
While our human body can store extra calories as lipids in adipose tissue, this capacity is limited. Obesity, a BMI larger than 30 kg/m2 is one of the fastest-growing health challenges in Europe and elsewhere. As of 2022, over half of the adult population (50.6%) in the EU was overweight, and around 17% were living with obesity (Eurostat, 2024; European Association for the Study of Obesity, 2025). Projections suggest that by 2030, nearly 30% of the EU population will be living with obesity (World Obesity Federation, 2023).
The obesity burden has far-reaching consequences for public health, as obesity is a key risk factor for an intricately linked cluster of comorbidities such as type 2 diabetes (T2D), cardiovascular diseases and certain types of cancer (Giroud et al., 2022). Currently, an estimated 80% of healthcare spending in the EU is related to noncommunicable diseases, associated with obesity (European Association for the Study of Obesity, 2025).
A critical driver of T2D is the body’s inability to respond properly to insulin, a hormone responsible for regulating blood glucose levels. After a meal, rising blood sugar is sensed by the beta cells of the endocrine pancreas, which respond by releasing insulin into the bloodstream. Insulin is the major anabolic hormone, targeting primarily adipose tissue, skeletal muscle and the liver. Among the actions of insulin is stimulating glucose uptake in the periphery and suppressing the build-up of new glucose molecules through a process taking place in the liver called gluconeogenesis.
In obesity, this finely tuned system becomes dysregulated. The problem lies in the body’s maladaptive response to sustained metabolic pressure caused by the prolonged caloric excess. Over time, insulin-sensitive tissues gradually lose their responsiveness to insulin—a condition known as insulin resistance. In response, the insulin system becomes chronically overactivated and increasingly inefficient. Ultimately, the pancreas can no longer meet the increased demand for insulin production, leading to persistently elevated blood glucose levels and setting the stage for the development of type 2 diabetes.
Insulin resistance: understanding why fat cells stop listening to hormonal cues
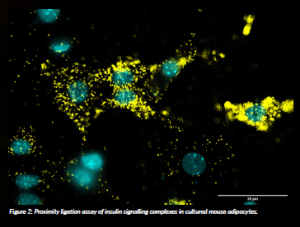
One major focus in our lab is to understand how the signalling pathways that regulate insulin sensitivity are disrupted in people with obesity. In healthy adipocytes, specialised fat cells, the amount of insulin receptors on the cell surface is tightly regulated to ensure proper responsiveness to insulin. In insulin resistance, however, this system breaks down: insulin receptors may not be trafficked properly or degraded, leading to impaired signalling and cellular dysfunction. We are using molecular biology, imaging and omics-based approaches to investigate the intracellular machinery that governs these processes. Our goal is to identify key components and mechanisms that are altered during the development of insulin resistance in adipocytes. By uncovering how these regulatory systems fail, we hope to contribute to the development of targeted strategies to restore insulin sensitivity and prevent or treat type 2 diabetes. In ongoing work, we are extending these findings to animal models to better understand their relevance in vivo.
Venturing into uncharted territory: adipocyte signalling relays and networks
In our work, we focus on the degradation of the insulin receptor, which is directly implicated in the development of insulin resistance. This includes finding new adaptor proteins for the insulin receptor as well as components of the machinery important for its internalisation and degradation. The goal is to use these key regulatory nodes to stabilise the insulin receptor and enhance insulin action in adipocytes. To understand the roles in adipocytes, we combined molecular biology, imaging and transcriptomic and proteomic approaches to investigate, in a controlled cell culture environment, how adipocyte function is affected when these molecules are depleted.
In healthy cells, insulin receptors are continuously recycled and degraded after insulin binding to maintain appropriate sensitivity. However, this regulatory mechanism is known to be disrupted in insulin resistance, contributing to defective signalling. Our findings suggest that new complexes exist that help maintain proper insulin receptor turnover and signalling in adipocytes. Together, these efforts contribute to a more detailed picture of how intracellular signalling is altered in obesity-related insulin resistance and may ultimately guide new treatment approaches for type 2 diabetes.
When the finely tuned signalling pathways inside adipocytes begin to break down, as in insulin resistance, the consequences extend far beyond impaired insulin action and glucose uptake. These disruptions affect the overall energy balance and trigger broader metabolic stress in adipocytes. Chronic low-grade inflammation, excess nutrients, and the accumulation of harmful metabolic byproducts place adipose tissue under strain, triggering a cascade of dysfunction that further amplifies insulin resistance and contributes to disease progression.
Under pressure from within: oxidative stress in obesity
Being stressed is often seen as an emotional state, but it stems from biochemical reactions. In obesity, our adipocytes are stressed. Oxidative stress—arising from an imbalance between the production of reactive oxygen species (ROS) and the body’s antioxidant defence systems—is a critical feature of the progression of obesity- related comorbidities. Adipocytes possess remarkable plasticity, and they expand in diameter when we gain weight. However, above a certain weight limit, this capacity is exhausted, and adipose tissue becomes poorly oxygenated and inflamed, creating conditions that promote excess ROS generation (Trayhurn, 2013). This oxidative stress damages cellular components such as proteins, lipids and DNA, and interferes with insulin signalling, contributing to metabolic dysfunction and the onset of type 2 diabetes.
Importantly, ROS are not merely byproducts of metabolic activity: they also act as signalling molecules, meaning that aberrant oxidative stress distorts critical cellular communication pathways and further interferes with metabolic regulation (Lennicke and Cochemé, 2021). Also, these redox disturbances are tightly connected to broader cellular imbalances. Mitochondrial function, glucose handling and lipid metabolism are all influenced by reinforcing cycles of stress. In this altered intracellular environment, cells face constant pressure to maintain homeostasis and increasingly rely on adaptive mechanisms to buffer metabolic strain. One such adaptation is the formation and remodelling of lipid droplets (LDs)—specialised intracellular organelles that serve as an energy reservoir, sequester potentially harmful lipids, mitigate oxidative damage and support cellular resilience under stress.
Lipid droplets: where fat meets function
We often think of lipids as something to get rid of, but within our cells, fat is stored in structures called lipid droplets that do much more than just stash extra energy for times of scarcity. Once viewed as passive fat stores, lipid droplets are now recognised as highly dynamic organelles with potential roles in buffering oxidative stress and preserving cellular integrity in metabolically challenged states (Walther and Farese, 2012; Olzmann and Carvalho, 2019).
In the context of obesity and diabetes, these organelles emerge as key players in managing oxidative stress. By sequestering excess fatty acids, LDs act as a protective sink, preventing lipid-induced toxicity and shielding mitochondria and membranes from oxidative damage (Bailey et al., 2015). This buffering capacity is particularly important in metabolically active tissues, such as adipose tissue, liver, and muscle, where fluctuations in nutrient availability and ROS levels are common. LDs have also been described as helping prioritise which lipids are stored, mobilised or catabolised under different stress conditions. This selective handling of lipid species may influence the availability of signalling lipids or dictate the cell’s susceptibility to lipid toxicity. LDs also serve as dynamic contact sites with mitochondria and the endoplasmic reticulum (ER), creating hubs for lipid exchange and metabolic coordination (Olzmann and Carvalho, 2019). These inter-organelle connections could enable LDs to spatially contain damage or redistribute metabolic burden during oxidative stress, particularly in tissues that face fluctuating nutrient and ROS levels. However, when LD homeostasis is disrupted, their protective function can falter.
Unleashed lipids cause cellular distress: what to do?
Impaired lipid droplet formation or turnover—whether due to excessive lipolysis, altered lipid composition or dysfunctional LD biogenesis—leads to the accumulation of free fatty acids in the cytosol. These unbuffered lipids are more likely to feed back into the oxidative stress cycle and further damage cellular components.
What are the consequences of a malfunctioning LD metabolism? In healthy cells, LDs help maintain insulin sensitivity by safely storing excess lipids and reducing the accumulation of lipid intermediates like ceramides or diacylglycerols that interfere with insulin pathways (Petersen and Shulman, 2017; Holland et al., 2007). However, when lipid storage capacity is overwhelmed or dysregulated, these harmful intermediates build up, activate stress kinases and inhibit insulin receptor signalling (Manieri and Sabio, 2015). This shows that changes in LD homeostasis are not only about storing extra fat, but also dictate the sensitivity to external cues. Thus, LDs sit at the intersection of nutrient management, oxidative stress buffering and hormone responsiveness—making their regulation a critical component of metabolic health.
Our research aims to uncover cellular and molecular mechanisms driving metabolic diseases, with a focus on insulin-sensitive organs such as adipose tissue. By understanding how signalling pathways become disrupted in obesity and T2D, we hope to contribute to a more detailed picture of how intracellular homeostasis is altered in obesity-related insulin resistance and identify new therapeutic strategies to restore insulin sensitivity and improve metabolic health. Healthy diets with high levels of plant-derived lipophilic vitamins and antioxidants, such as vitamin E or polyphenols, are a good strategy to combat metabolic stress from within.
References
Bailey, A.P., Koster, G., Guillermier, C., Hirst, E.M.A., MacRae, J.I., Lechene, C.P., Postle, A.D. and Gould, A.P. (2015) ‘Antioxidant role for lipid droplets in a stem cell niche of Drosophila’, Cell, 163(2), pp. 340–353. doi: 10.1016/j.cell.2015.09.020.
Eurostat (2024) Overweight and obesity – BMI statistics – Statistics Explained. Available at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Overweight_and_obesity-BMI_statistics.
European Association for the Study of Obesity (2025) EASO policy recommendations for EU action to address obesity. Available at: https://easo.org/wp-content/uploads/2025/04/2025-policy-recommendations-final.pdf.
Giroud, M., Jodeleit, H., Prentice, K.J. and Bartelt, A. (2022) ‘Adipocyte function and the development of cardiometabolic disease’, Journal of Physiology, 600(5), pp. 1189–1208. doi: 10.1113/JP281979.
Holland, W.L., Brozinick, J.T., Wang, L.P., Hawkins, E.D., Sargent, K.M., Liu, Y., Narra, K., Hoehn, K.L., Knotts, T.A., Siesky, A., Nelson, D.H., Karathanasis, S.K., Fontenot, G.K., Birnbaum, M.J. and Summers, S.A. (2007) ‘Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturatedfat, and obesityinduced insulin resistance’, Cell Metabolism, 5(3), pp. 167–179. doi: 10.1016/j.cmet.2007.01.002.
Lennicke, C. and Cochemé, H.M. (2021) ‘Redox metabolism: ROS as specific molecular regulators of cell signaling and function’, Molecular Cell, 81(18), pp. 3691–3707. doi: 10.1016/j.molcel.2021.08.018.
Manieri, E. and Sabio, G. (2015) ‘Stress kinases in the modulation of metabolism and energy balance’, Journal of Molecular Endocrinology, 55(2), pp. R11–R22. doi: 10.1530/JME-15-0146.
Olzmann, J.A. and Carvalho, P. (2019) ‘Dynamics and functions of li- pid droplets’, Nature Reviews Molecular Cell Biology, 20(3), pp. 137–155. doi: 10.1038/s41580-018-0085-z.
Petersen, M.C. and Shulman, G.I. (2017) ‘Roles of diacylglycerols and ceramides in hepatic insulin resistance’, Trends in Pharmacological Sciences, 38(7), pp. 649–665. doi: 10.1016/j.tips.2017.04.004.
Trayhurn, P. (2013) ‘Hypoxia and adipose tissue function and dysfunction in obesity’, Physiological Reviews, 93(1), pp. 1–21. doi: 10.1152/physrev.00017.2012.
Walther, T.C. and Farese, R.V. (2012) ‘Lipid droplets and cellular lipid metabolism’, Annual Review of Biochemistry, 81, pp. 687–714. doi: 10.1146/annurev-biochem-061009-102430.
World Obesity Federation (2023) World Obesity Atlas 2023. Available at: https://s3-eu-west-1.amazonaws.com/wof-files/World_Obesity_ Atlas_2023_Report.pdf.
Project summary
Physical activity and exercise have beneficial effects on overall fitness and health. However, exercise-induced increases in metabolism require specific molecular adaptation of the muscle cells. This project aims to understand the molecular processes in the muscle during the adaptation to cold, exercise and obesity, thereby defining novel mechanisms of protein homeostasis with regard to the gene switch Nfe2l1.
Project lead
Alexander Bartelt is the Else Kröner Fresenius Professor and Chair of Translational Nutritional Medicine at the Technical University of Munich (TUM) Campus Weihenstephan. He studied biochemistry at the University of Hamburg, earning his diploma in 2007 and his PhD with honours in 2010. Following his doctoral studies, he completed postdoctoral training at Harvard University, USA, in 2013. He then returned to Germany as Professor of Cardiovascular Metabolism at the Institute for Cardiovascular Prevention (IPEK), Ludwig Maximilians University Munich, a position he held from 2019 to 2024. The Bartelt lab is dedicated to understanding the basic principles of metabolic adaptation as well as the molecular pathology of cardiometabolic diseases. Over the years, Dr Bartelt’s contributions have been recognised by national and international awards and distinctions.
Project partners
This project is based at the Institute for Cardiovascular Prevention (IPEK) at the LMU Munich in collaboration with the Helmholtz Diabetes Center Munich.
Contact details
Univ.-Prof. Dr Alexander Bartelt
Email: alexander.bartelt@tum.de
Anna Jung, MSc
Email: anna.jung@tum.de
Alba Mena Gómez, MSc
Email: alba.mena@tum.de
Web: www.mls.ls.tum.de/tnm
Bluesky: @barteltlab.bsky.social
FUNDING
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 852742.
Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the ERC. Neither the European Union nor the granting authorities can be held responsible for them.