Prof. Luca Unione, CIC bioGUNE
Every human cell is covered by a dense coat of glycans (sugars, carbohydrates). Sugars are the first molecules that viruses encounter when they approach host cells. Many viruses encode lectins to adhere to the outer glycan layer and start infection. In this scenario, glycoscience holds a tremendous potential for combating infections. Glyco13Cell propose a groundbreaking strategy to untangle the key glycan’s molecular interaction features on cells and answer fundamental questions related to sugar structure, presentation, dynamics and, eventually, function.
Glycoconjugates, in the form of cell surface glycoproteins and glycolipids, are involved in maintaining cell homeostasis and health (Reily et al., 2019). One of the key roles of glycans is to mediate interactions at the virus-host interface, controlling viral spread and/or activation of the host immune system (Thompson, de Vries and Paulson, 2019). Thinking of our cells as a tennis ball, the glycans would be the felt. As we cannot catch the ball without touching the felt, pathogens cannot colonise cells without crossing the dense forest of glycans.
Evolution has endowed pathogens with arms to cross the barrier. Numerous viruses carry virally encoded lectins (glycan binding proteins) on their surface to be used as keys to gain entry into their target cells, making the glycan coat the front line of the battle. Examples include human influenza A, B and C, human parainfluenza 3 and 5, and most coronaviruses, among others (Schwegmann-Wessels and Herrler, 2006).
Yet, glycans on the host cell surface are structurally very diverse (Varki, 2006). During evolution, glycan biosynthesis diverged and, as a result, the structures of glycans differed considerably, even between closely related species, tissues and cells. These differences are partly driven by pathogenic pressure to hamper cross-species transmission (Le Pendu, Nyström and Ruvoën-Clouet, 2014).
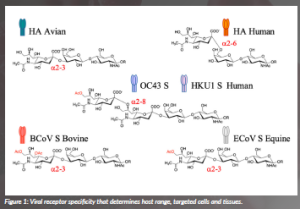
The selectivity of virolectins for specific glycan structures critically determines host range, targeted tissues and cells, and pathogenesis (Figure 1).
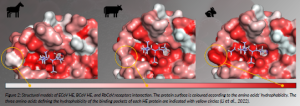
Certain corona-, toro- and influenza C and D viruses show host-specific receptor recognition that is related to O-acetylation patterns and sialic acid glycosidic linkage types (Li et al., 2021). O-acetylation is the most common modification of sialic acid, which can occur at the 4-, 7-, 8- and/ or 9-positions to give rise to partially O-acetylated variants. The expression of these glycotopes is regulated in a tissue- and cell-specific manner; thus, different host species express different repertoires of O-acetylated sialosides. The molecular basis by which these viruses recognise specific O-acetylated sialosides is poorly understood, and it is unknown how viruses have evolved to recognise specific O-acetylated sialosides expressed by their host. Recently, we described a chemoenzymatic approach that readily provided a portfolio of acetylated sialoglycan variants. Those molecules were used to examine the ligand requirements of the coronaviral hemagglutinin-esterases (HEs) lectin domain. Atomic-level NMR and molecular modelling of the complexes of the HE of different host species with the corresponding acetylated sialosides revealed that binding is governed by the complementarity between the acetyl moieties of the sialosides and the hydrophobic patches of the HE lectins with stringent requirements for the precise spatial arrangement of these elements (Li et al., 2022). The results demonstrated that coronaviruses infecting different species have adapted their HE specificity by the incorporation of hydrophobic or hydrophilic elements to modulate acetyl ester recognition (Figure 2).
Although the diversity of glycome limits cross-species transmission, occasionally, viruses cross the species barrier, sparking pandemics. The influenza A virus uses hemagglutinin (HA) lectins to bind to cell surface glycans decorated with sialic acid receptors. These receptors come in different forms and have different distributions in birds, humans and other mammalian species. The human influenza A virus attaches via the HA protein to glycans on the host cell surface carrying terminal α2,6-linked sialic acid moieties Neu5Ac(a2,6)Gal (Rogers and Paulson, 1983). In contrast, its ancestor, the HA of avian viruses, exhibits specificity for the α2,3-linked isomer (Shinya et al., 2006). Therefore, influenza spillover from bird to human requires mutations at the HA receptor-binding domain to switch receptor binding to glycans with α2,6-linked sialic acids instead of α2,3- analogues. In previous human pandemics that originated from avian viruses, only two amino-acid mutations in the receptor-binding pocket of the HA were sufficient to produce a switch from avian-type to human-type specificity.
Since the introduction of influenza A viruses in humans, the viral hemagglutinin protein has continuously evolved under the pressure of immune escape. During the last six decades, H3N2 influenza A viruses have infected humans and escaped neutralisation by antibodies induced by vaccination or previous infections by accumulating mutations in the HA protein. Yet, antigenic changes in and around the receptor-binding site (RBS) of HAs not only avoid antibody neutralisation but might also impact the interactions with sialylated glycans and, eventually, compromise effective viral adhesion. Preserving this intricate balance between antigenic divergence and receptor binding is not evident. This process, known as antigenic drift, recently resulted in the strict recognition of α2,6-linked sialoside presented onto extended oligosaccharide (Peng et al., 2017). To deal with the molecular details of such interaction through NMR spectroscopy, we embarked on synthesising complex N-glycans bearing specific 13C-labels in strategic positions.
The preparation of the isotopomers was critical for the STD NMR experiments and for uncovering the contributions of each monosaccharide for binding to various HAs. This type of NMR experiment is, in fact, a powerful approach to characterise glycan interactions with proteins at the atomic level. Ligand protons in close proximity to the protein receive saturation, resulting in strong STD signals. On the other hand, ligand protons that are more remote or do not engage with the protein exhibit weak or no STD signals. Large glycans suffer, however, from very low chemical shift dispersion of their 1H NMR signals, precluding detailed binding studies by 1H-STD NMR. This is especially problematic when glycans present repeating units, such as poly- LacNAc chains, as these have almost indistinguishable 1H chemical shifts. The presence of the 13C-labels breaks the NMR chemical shift degeneracy that occurs in the non-labelled compound without introducing any artificial probe that may interfere with its binding properties. 13C probes were then used to unravel the contribution of each monosaccharide to the HA protein binding (Unione et al., 2024).
The NMR data, in combination with in silico and mutagenesis studies, demonstrate that, along its antigenic evolution, mutations distal to the receptor-binding domain of HA create an extended binding site that can directly interact with the extended LacNAc chain of glycans. The results showed that for the original pandemic A/H3N2 human virus (HK68), the poly-LacNAc moiety does not contribute to binding, whereas, for later strains, site-specific mutations close to its glycan binding site enable further interactions with the extended glycan chain. For the evolutionarily early NL91 HA, these interactions are mostly limited to the sialic acid-linked galactoside, while the more contemporary NL03 HA recognises almost the entire poly-LacNAc chain (Figure 3).
Although this in-solution experimental approach demonstrated the participation of the underlying oligosaccharide to virolectin binding and affinity, an open question remains: how accessible are those glycans on the cell surface? Glyco13Cell aims to investigate glycan/virolectin recognition events at the atomic level in living cells. To address this question, Glyco13Cell proposes creating glyco-defined cells that incorporate isotopically labelled glycans on their surface to study glycan/virolectin interactions at atomic resolution under near-to-in vivo conditions by NMR. The project makes use of an interdisciplinary approach that conjugates cellular and molecular biology, chemical synthesis and on-cell NMR (Figure 4). Specific glycan/ virolectin-mediated processes will be investigated: the human influenza A virus (virolectin: Hemagglutinin [HA]) and human coronaviruses [CoVs] OC43 and HKU1 [virolectin spike protein]).
Glyco13Cell will chemically remodel glycans directly on the cell surface. The modified glycans will contain NMR-active probes to decode their biological role at the highest possible resolution, from chemistry to biology and beyond. The use of isotopes brings three key advantages:
- preserve presentation,
- do not interfere with binding, and
- allow NMR-based studies to unravel detection and interactions.
The proposed strategy will narrow the observed differences between in vitro and in vivo results, paving the way for the development of novel glycan-based tools for predicting, diagnosing and treating infectious diseases.
References
Broszeit, F., van Beek, R.J., Unione, L., Bestebroer, T.M., Chapla, D., Yang, J.Y., Moremen, K.W., Herfst, S., Fouchier, R.A.M., de Vries, R.P. and Boons, G.-J. (2021) ‘Glycan remodeled erythrocytes facilitate antigenic characterization of recent A/H3N2 influenza viruses’, Nature Communications, 12(1), 5449. doi: 10.1038/s41467-021-25713-1.
Le Pendu, J., Nyström, K. and Ruvoën-Clouet, N. (2014) ‘Host-pathogen co-evolution and glycan interactions’, Current Opinion in Virology, 7, pp. 88–94. doi: 10.1016/j.coviro.2014.06.001.
Li, Z., Lang, Y., Liu, L., Bunyatov, M.I., Sarmiento, A.I., de Groot, R.J. and Boons, G.-J. (2021) ‘Synthetic O-acetylated sialosides facilitate functional receptor identification for human respiratory viruses’, Nature Chemistry, 13, pp. 496–503. doi: 10.1038/s41557-021-00655-9.
Li, Z., Unione, L., Liu, L., Lang, Y., de Vries, R.P., de Groot, R.J. and Boons, G.-J. (2022a) ‘Synthetic O-acetylated sialosides and their acetamido- deoxy analogues as probes for coronaviral hemagglutinin-esterase recognition’, Journal of the American Chemical Society, 144(1), pp. 424–435. doi: 10.1021/jacs.1c10329.
Li, Z., Liu, L., Unione, L., Lang, Y., de Groot, R.J. and Boons, G.J. (2022b) ‘Synthetic O-Acetyl-N-glycolylneuraminic Acid Oligosaccharides Reveal Host-Associated Binding Patterns of Coronaviral Glycoproteins’, ACS Infectious Diseases, 8(5), pp. 1041–1050. doi: 10.1021/acsinfecdis.2c00046.
Peng, W., de Vries, R.P., Grant, O.C., Thompson, A.J., McBride, R., Tsogtbaatar, B., Lee, P.S., Razi, N., Wilson, I.A., Woods, R.J. and Paulson, J.C. (2017) ‘Recent H3N2 viruses have evolved specificity for extended, branched human-type receptors, conferring potential for increased avidity’, Cell Host & Microbe, 21(1), pp. 23–34. doi: 10.1016/j.chom.2016.11.004.
Reily, C., Stewart, T.J., Renfrow, M.B. and Novak, J. (2019) ‘Glycosylation in health and disease’, Nature Reviews Nephrology, 15(6), pp. 346–366. doi: 10.1038/s41581-019-0129-4.
Rogers, G.N. and Paulson, J.C. (1983) ‘Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin’, Virology, 127(2), pp. 361–373. doi: 10.1016/0042-6822(83)90150-2.
Schwegmann-Wessels, C. and Herrler, G. (2006) ‘Sialic acids as receptor determinants for coronaviruses’, Glycoconjugate Journal, 23(1–2), pp. 51–58. doi: 10.1007/s10719-006-5437-9.
Shinya, K., Ebina, M., Yamada, S., Ono, M., Kasai, N. and Kawaoka, Y. (2006) ‘Avian flu: influenza virus receptors in the human airway’, Nature, 440(7083), pp. 435–436. doi: 10.1038/440435a.
Thompson, A.J., de Vries, R.P. and Paulson, J.C. (2019)’ Virus recognition of glycan receptors’, Current Opinion in Virology, 34, pp. 117–129. doi: 10.1016/j.coviro.2019.01.004.
Tomris, I., Unione, L., Nguyen, L., Zaree, P., Bouwman, K.M., Liu, L., Li, Z., Fok, J.A., Ríos Carrasco, M., van der Woude, R., Kimpel, A.L.M., Linthorst, M.W., Kilavuzoglu, S.E., Verpalen, E.C.J.M., Caniels, T.G., Sanders, R.W., Heesters, B.A., Pieters, R.J., Jiménez-Barbero, J., Klassen, J.S., Boons, G.-J. and de Vries, R.P. (2023) ‘SARS-CoV-2 Spike N-Terminal Domain engages 9-O-acetylated α2-8-linked sialic acids’, ACS Chemical Biology, 18(5), pp. 1180–1191. doi: 10.1021/acschembio.3c00066.
Unione, L., Moure, M.J., Lenza, M.P., Oyenarte, I., Ereño-Orbea, J., Ardá, A. and Jiménez-Barbero, J. (2022) ‘The SARS-CoV-2 Spike Glycoprotein directly binds exogeneous sialic acids: a NMR view’, Angewandte Chemie (International ed. in English), 61(18), e202201432. doi: 10.1002/anie.202201432.
Unione, L., Ammerlaan, A.N.A., Bosman, G.P., Uslu, E., Liang, R., Broszeit, F., van der Woude, R., Liu, Y., Ma, S., Liu, L., Gómez-Redondo, M., Bermejo, I.A., Valverde, P., Diercks, T., Ardá, A., de Vries, R.P. and Boons, G.-J. (2024) ‘Probing altered receptor specificities of antigenically drifting human H3N2 viruses by chemoenzymatic synthesis, NMR, and modeling’, Nature Communications, 15(1), 2979. doi: 10.1038/s41467-024-47344-y.
Varki, A. (2006) ‘Nothing in glycobiology makes sense, except in the light of evolution’, Cell, 126(5), pp. 841–845. doi: 10.1016/j.cell.2006.08.022.
Zeng, Q., Langereis, M.A., van Vliet, A.L., Huizinga, E.G. and de Groot, R.J. (2008) ‘Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution’, Proceedings of the National Academy of Sciences of the United States of America, 105(26), pp. 9065–9069. doi: 10.1073/pnas.0800502105.
PROJECT NAME
GLYCO13CELL
PROJECT SUMMARY
Isotopically labelling of cell surface glycans to illuminate infectious processes at atomic resolution is a multidisciplinary project from fundamental to applied science, with high potential for translation. The chemically modified cells will be used to investigate their interactions with viral lectins and uncover the roles that glycan structure, presentation and dynamics play in viral infections. Understanding the underlying interaction mechanisms under near-to-in-vivo conditions will pave the way for developing novel glycan-based tools for predicting, diagnosing and treating infectious diseases. The generated knowledge will inspire the design of glycan-based inhibitors of infection.
PROJECT PARTNERS
GLYCO13CELL is based at the Center for Cooperative Research in Biosciences (CIC bioGUNE, Bilbao), taking advantage of the expertise in chemical immunology, structural and cell biology of viruses, cancer immunology and immunotherapy, and computational chemistry. The infrastructure includes advanced equipment for NMR, now recognised as Singular Scientific Infrastructure in Spain, cryo-electron microscopy (in collaboration with BREM), and X-ray diffraction, facility for mammalian cell cultures, fully equipped chemical synthesis labs, genomics, proteomics and metabolomics platforms.
PROJECT LEAD PROFILE
Luca Unione is Ikerbasque Associate Professor and Principal Investigator in the Chemical Glycobiology lab, leading the research area of glycan in infectious diseases. He received his PhD in Madrid (2016). Earlier in his career, he worked at Napoli, Madrid, Bilbao, Utrecht and Bilbao again.
PROJECT CONTACT
Bizkaia Technology Park, Building 800; 48160 Derio, Spain,
Tel: 4209 / 944 061 321
Email: lunione@cicbiogune.es
Web: www.cicbiogune.es/people/lunione
www.ikerbasque.net/es/luca-unione
FUNDING
This project has received funding from the European Research Council (ERC) under the European Union’s ERC Starting Grant 2023 under grant agreement No. 101117639.
Figures
Figure 1: Viral receptor specificity that determines host range, targeted cells and tissues.
Figure 2: Structural models of ECoV HE, BCoV HE, and RbCoV:receptors interaction. The protein surface is coloured according to the amino acids’ hydrophobicity. The three amino acids defining the hydrophobicity of the binding pockets of each HE protein are indicated with yellow circles (Li et al., 2022).
Figure 3: Structural models of the HAs:receptor interactions for the hemagglutinin proteins of Hong Kong 1968 (HK68), The Netherlands 1991 (NL91) and The Netherlands 2003 (NL03) as derived from the NMR binding experiments with the 13C-isotopically labelled N-glycans (Unione et al., 2024).
Figure 4: Schematic representation of Glyco13Cell strategy. Naturally occurring glycans on cell surfaces are intrinsically heterogeneous. By combining cellular biology and chemical tools, Glyco13Cell will re-model those glycans to produce homogeneous structures. NMR-active probes will be introduced. The molecular features of the interaction between the synthesised glycans and virolectins will be monitored directly on living cells.