Hiroki Shibuya, University of Gothenburg, Sweden
DNA damage and BRCA2
DNA is constantly damaged by endogenous factors (e.g. hydrolytic and oxidative reactions and DNA replication errors) and exogenous agents (e.g. UV radiation, ionising radiation, and mutagenic chemicals). Such DNA lesions threaten genomic integrity and predispose cells to cancer development (Jackson and Bartek, 2009). The DNA is composed of double helixes and, among the different types of DNA lesions, damage that cuts both DNA strands simultaneously, called DNA double-strand breaks (DSBs), is considered the most hazardous form of DNA damage.
Breast cancer susceptibility gene 2 (BRCA2)was identified in 1995 and has been the subject of intensive research over the past 25 years. BRCA2 is a potent cancer suppressor gene and acts as a guardian of genomic integrity (Fradet-Turcotte et al., 2016). The molecular function of the BRCA2 protein is to repair the DSBs in a high-fidelity (i.e. error-free) manner via the homologous recombination (HR) pathway. Germline mutations in the BRCA2 gene make individuals susceptible to DNA damage and predispose them to various cancers, including ovarian and breast cancers. In fact, women carrying BRCA2 mutations have up to a 70 per cent risk of developing breast cancer by age 80.
Despite the importance from a clinical perspective, the molecular functions and regulations of BRCA2 are not yet fully understood. Significantly, the role of BRCA2 in germ cells, where the induction of programmed DNA breaks is an integral mechanism essential for normal germ cell production, has been largely overlooked.
Meiosis
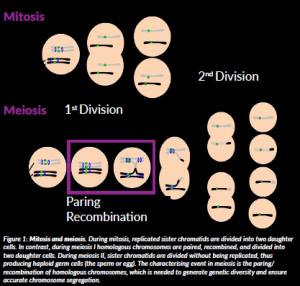
Meiosis is a specialised form of cell division for germ cell production. In normal somatic cells, there is only one cycle of cell division after a DNA replication. In contrast, there are two successive rounds of cell division in meiosis after DNA replication, which result in the generation of haploid germ cells (sperms and eggs) containing only half the number of chromosomes (Figure 1). The characteristic event in meiosis is the HR between homologous chromosomes (i.e. paternal and maternal chromosomes).
Intentional induction of abundant DNA damages in germ cells
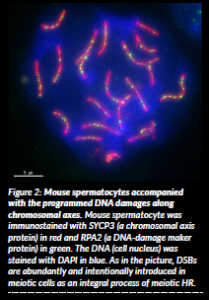
DSBs are hazardous forms of DNA damage and undesired products to the somatic cells. However, DSBs are intentionally and abundantly introduced in germ cells undergoing meiosis despite their potential threat to genomic integrity (Figure 2). This programmed induction of abundant DSBs and its efficient repair in meiotic cells are needed to exchange DNA strands between homologous chromosomes as the initial step of meiotic HR. In the meiotic HR, the DNA from paternal and maternal chromosomes is mixed, generating totally new chromosomes and promoting genetic diversity. The physical connection between paternal and maternal chromosomes is achieved via meiotic HR, which is needed for the proper segregation of these chromosomes. If the meiotic HR goes awry, chromosome missegregation occurs. The resultant germ cells will have an abnormal number of chromosomes, which cause human infertility, birth defects, Down syndrome, and Turner syndrome.
Discovery of meiosis-specific BRCA2 co-factors
During our ERC-funded research, we screened for novel proteins involved in meiotic HR and identified the meiosis- specific BRCA2 co-factors that we named MEILB2 (meiotic localiser of BRCA2) and BRME1 (BRCA2 and MEILB2-associating protein 1) (Zhang et al., 2019; Zhang et al., 2020). Both MEILB2 and BRME1 are specifically functioning in germ cells. We looked at their function by generating gene knockout mice. Without MEILB2 or BRME1, the BRCA2 function is attenuated, and meiotic DSBs are not repaired correctly, leading to defects in meiotic HR, which then causes germ cell death and male sterility.
By collaborating with a research group at the University of Michigan, we purified the protein complex of MEILB2-BRCA2 and solved the crystal structure (Pendlebury et al., 2021). Interestingly, MEILB2 binds to the functionally uncharacterised domain of BRCA2, which we termed the MEILB2- binding domain. The binding of MEILB2 to BRCA2 promotes the tight dimerisation of BRCA2 molecules, which is likely needed for the meiosis-specific hyperactivation of BRCA2. Our findings now suggest that the MEILB2-binding domain of BRCA2 might be a hotspot for discovering mutations related to infertility.
Future perspectives
The misregulation of meiotic HR is related to human infertility, azoospermia, and birth defects. The analysis of BRCA2-MEILB2- BRME1’s molecular function in meiotic HR will increase our understanding of the aetiology of such human diseases. In fact, a recent study identified mutations in human MEILB2 genes in families with primary ovarian insufficiency (Felipe- Medina et al., 2020). Certain links have also been identified between aberrant expression of MEILB2 and BRME1 genes and cancer development. Opposite to their roles as BRCA2 activators in germ cells, MEILB2 and BRME1 seem to function as an inhibitory factor of BRCA2 when overexpressed in somatic cells (Zhang et al., 2020; Sato et al., 2020). The ectopic overexpression of MEILB2 and BRME1 in somatic cells inhibit the function of BRCA2, leading to the misregulation of key downstream DSB repair factors such as the RAD51 protein. Indeed, MEILB2 and BRME1 genes are frequently upregulated in human cancers (Zhang et al., 2020; Sato et al., 2020).
How MEILB2 and BRME1 impair somatic BRCA2 function and contribute to cancer development is an exciting area for further exploration. A number of sporadic cancers showed similar phenotypes seen in familial BRCA2 mutated cancers even without the BRCA2 mutation. In such sporadic cancer cases, the overexpression of BRCA2 partner proteins can be a driving force for cancer development. In the future, this could be interesting as a route towards helping diagnose these cancers at an earlier stage or potentially to identify a new target of cancer therapy.
Images
Figure 1: Mitosis and meiosis. During mitosis, replicated sister chromatids are divided into two daughter cells. In contrast, during meiosis I homologous chromosomes are paired, recombined, and divided into two daughter cells. During meiosis II, sister chromatids are divided without being replicated, thus producing haploid germ cells (the sperm or egg). The characterising event in meiosis is the paring/ recombination of homologous chromosomes, which is needed to generate genetic diversity and ensure accurate chromosome segregation.
Figure 2: Mouse spermatocytes accompanied with the programmed DNA damages along chromosomal axes. Mouse spermatocyte was immunostained with SYCP3 (a chromosomal axis protein) in red and RPA2 (a DNA-damage maker protein) in green. The DNA (cell nucleus) was stained with DAPI in blue. As in the picture, DSBs are abundantly and intentionally introduced in meiotic cells as an integral process of meiotic HR.
References
Felipe-Medina, N. et al. (2020) ‘A missense in HSF2BP causing primary ovarian insufficiency affects meiotic recombination by its novel interactor C19ORF57/BRME1’, eLife, 9. doi: 10.7554/elife.56996.
Fradet-Turcotte, A. et al. (2016) ‘BRCA2 functions: from DNA repair to replication fork stabilization’, Endocrine-Related Cancer, 23(10), T1-T17. doi: 10.1530/ERC-16-0297.
Jackson, S.P. and Bartek, J. (2009) ‘The DNA-damage response in human biology and disease’, Nature, 461(7267), pp.1071–1078. doi: 10.1038/nature08467.
Pendlebury, D.F. et al. (2021) ‘Structure of a meiosis-specific complex central to BRCA2 localization at recombination sites’, Nature Structural & Molecular Biology, 28, pp. 671–680. doi: 10.1038/s41594-021-00635-0.
Sato, K. et al. (2020) ‘HSF2BP negatively regulates homologous recombination in DNA interstrand crosslink repair’, Nucleic Acids Research, 48(5), pp.2442–2456. doi: 10.1093/nar/gkz1219.
Zhang, J.J. et al. (2019) ‘A meiosis-specific BRCA2 binding protein recruits recombinases to DNA double- strand breaks to ensure homologous recombination’, Nature Communications, 10(722). doi: 10.1038/s41467-019-08676-2.
Zhang, J.J. et al. (2020) ‘The BRCA2-MEILB2-BRME1 complex governs meiotic recombination and impairs the mitotic BRCA2-RAD51 function in cancer cells’, Nature Communications, 11(1). doi: 10.1038/s41467-020-15954-x.
Article summary
Project name
Meiotic Telomere
Project summary
Plenty of unique and sophisticated events happen in germ cells to faithfully transmit paternal and maternal chromosomes to the next generation. Errors in this process will cause human birth defects, infertility, aneuploidy, and various genetic disorders. We are trying to understand the regulation of chromosome dynamics in germ cells by employing a combination of genetic, molecular, cytological, and biochemical approaches.
Project lead
Hiroki Shibuya is an Assistant Professor in the Department of Chemistry and Molecular Biology, University of Gothenburg, Sweden. He obtained PhD at the University of Tokyo, Japan, in 2014. After his PhD, Shibuya worked at Harvard Medical School, USA, as a Human Frontier Scientific Program Long- term Fellowship Postdoc.
Contact details
Hiroki Shibuya
Medicinaregatan 9E, SE-41390, GOTHENBURG, Sweden
Tel: +46 70 006 07 79
Email: hiroki.shibuya@gu.se
Web: shibuyahiroki.com
Funding
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No.801659.