Silvia Lasala, Université de Lorraine, France
Concept
The present role of thermodynamic cycles
Concept
The present role of thermodynamic cycles
Thermodynamic cycles constitute the backbone structure of fossil-fuelled and renewable thermal power systems, refrigerators and heat pumps (Carnot, 1824). In thermodynamic cycles, input energies are converted into output useful energy forms (work or heat) by means of an energy carrier, an inert working fluid, which undertakes cycles of thermodynamic transformations. Power cycles have been dominating global electricity production, and it is expected that refrigerators and heat pumps will represent one of the future major electricity consumers. Improvements of their backbone-thermodynamic structure thus play a crucial role in achieving energy-policy objectives addressing climate change and reducing air pollution, such as the increase of the efficiency of energy use and the deployment of renewable technologies.
Performance improvement by working fluid selection
To increase the performance of thermodynamic cycles, research mainly focuses on improving their unit operations, optimising the networking of these components, and optimal selection of a working fluid crossing the whole cycle. Among these possible measures, the choice of the optimal working fluid represents the core of the process of adaptation of conventional fossil-fuelled thermal engine configurations to exploit lower-grade renewable and waste heat sources and the primary action to reduce the environmental impact of heat pumps.
Getting stuck on inert working fluids
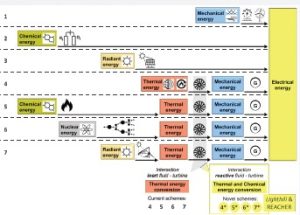
Only inert working fluids (pure fluids or mixtures) are currently employed (see Figure 1). Despite their optimal selection, the thermal efficiency of these energy conversion systems remains far from the maximum achievable ones, dictated by the Carnot limit (Cullen and Allwood, 2010).
Involving chemical energy in thermodynamic cycles: a hint for scientific progress
A breakthrough idea was suggested by Lighthill (1957): he proposed to convert the chemical energy like the “large energy change involved in dissociating gases” into work. Practically, he put forward the idea to use reactive working fluids, instead of inert ones, in closed power cycles. As shown in Figure 2, differently from current energy conversion systems for electricity production (Figure 2, 1–7), which are formed by one or more devices being the place of distinguished energy-type transformations, Lighthill’s idea consists of a novel energy conversion process, where the transformation of thermal energy into mechanical one is made possible by the concurrent thermal and chemical conversion of the energetic state of a reactive fluid, all along a closed “thermo-chemical” cycle (Figure 2, 4*–7*).
REACHER: why now?
Starting from the 1960s, some researchers have investigated the performances of classical power cycles operating with some reactive working fluids, mainly N2O4(g) ⇆ 2NO2(g) ⇆ 2NO(g)+O2(g) (Krasin and Nesterenko, 1971; Bradley, 1976; Angelino, 1979; Sorokin, 1979; Kesavan and Osterle, 1982). At the time of these studies, the potential of inert fluids was not yet totally investigated. The more advanced idea of using reactive fluids was not fully appreciated and thus remained scientifically unexplored. Now that the use of inert fluids reveals limitations, REACHER intends to revive Lighthill’s idea.
Promising preliminary results with optimal fictive reactive fluids
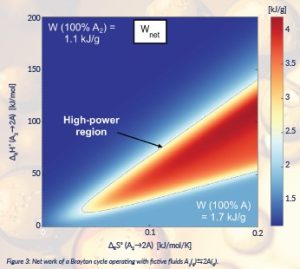
A theoretical investigation has recently been published (Lasala et al., 2021), based on an original chemical product design approach, where the ‘product’ is a fictive gaseous and equilibrated chemical reaction and ‘equilibrated reaction’ means that kinetics is negligible and, hence, the reaction composition is dictated by thermodynamic equilibrium. Indeed, the impact of slow kinetics would lead to non-stationary cycles and performances comprised of those between the ones of inert and of equilibrated-reactive fluid. This work demonstrates that, for comparable inert fluids (e.g. pure reactants or pure products or their inert mixtures), the use of specific reaction features (stoichiometry, reference standard enthalpy (∆RH°) and entropy (∆RS°) of reaction) may allow much higher performances to be achieved, in even classical (non-optimal) cycle configurations. Figure 3 shows how the net work (Wnet) of a closed Brayton cycle varies with the different couples of ∆RH° and ∆RS° of the fictive equilibrated reactive fluid A2 D 2A. In the blue regions, (∆RH°, ∆RS°)-reaction features imply an inert-type behaviour along the cycle (inert reactants or products). By comparing, in Figure 3, the net work resulting from the use of chemically reactive fluids (red region) with the one of inert fluids (blue regions), it is possible to observe that the former more than double the net work. Although only theoretical, such a preliminary work has sampled the ground-breaking potential of Lighthill’s idea, presented the main barrier to be overcome (searching for suitable real reactive fluids) and the ways for further efficiency improvement (defining new optimal cycle configurations).
REACHER’s goals: a novel chemical design path for a novel chemical-energy conversion scheme
REACHER seeks to achieve two targets. The primary target is the design of equilibrated reactive fluids through an original thermodynamic and kinetic predictive methodology using the group contribution methods, quantum chemistry-based computational aided molecular design and machine learning. This will enable the secondary target to explore a new energy science domain based on the exploitation of chemical energy in closed thermodynamic cycles (secondary-level target).
Methodology
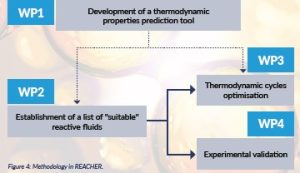
REACHER consists of four work packages (WPs) (Figure 4).
WP1 – Development of a computational tool for the prediction of thermodynamic properties
The achievement of REACHER’s targets relies on the realisation of a reliable tool to predict thermodynamic properties of reactive fluids. That will require the preliminary selection, improvement and implementation of a predictive equation of state based on group contribution method (Gmehling, Constantinescu and Schmid, 2015) for mixture modelling and of algorithms for chemical equilibrium calculation.
WP2 – Establishment of a list of ‘suitable’ reactive fluids
The objective of this work package is the definition of a list of reactions considered suitable for the set of studied applications and the characterisation of their thermodynamics and kinetics. That consists in the implementation of the following steps:
- Definition of thermochemical criteria (ranges of enthalpy and entropy of reaction) for reaction searching and design
- Search for and design of reactions fulfilling pre-defined thermodynamic criteria
- Characterisation of the kinetics of listed fluids and further selection of fast reactions.
WP3 – Optimisation of thermodynamic cycles operating with reactive fluids
Considering a specific application of power, heating or cooling, the optimisation of the overall system (fluid and cycle architecture) consists of two steps. Firstly, a process design method will be applied to determine the optimal cycle architecture for each fluid of the list defined in WP2. Secondly, a comparison between all these optimised systems, on the basis of their performance indicators, will provide the best fluid-architecture system. Finally, the same process design method will be applied, considering some inert working fluids. Optimal solutions obtained with inert and reactive working fluids will be finally compared to quantify REACHER’s gain.
WP4 – Observing the transformation of thermal and chemical energy into work
This part of the project is devoted to the experimental validation of the energy conversion process undergone by two preliminary selected reactive fluids in the expansion taking place in a micro-axial turbine. The composition change across the turbine will be measured by Raman spectroscopy techniques.
Impact
Main scientific impacts
The breakthrough understanding of the fundamental relation between reactive fluid characteristics and their energy transformations will open a new scientific research field on the exploitation of chemical energy.
The application of cutting-edge methods for discovering and characterising new fluids and reactions will contribute to addressing one of the biggest scientific challenges (Grossmann, 2004): product discovery and characterisation.
This project will contribute to improving the analysis of spectroscopy measurements used to quantify fluid composition, an open research field.
Main societal impact
If the expected performances of analysed thermo-chemical cycles are confirmed, the outcomes of this project will allow the enhanced exploitation of available waste heat and renewable thermal energy sources by means of small, powerful and efficient machines.
References
Angelino, G. (1979) ‘Performance of N2O4 gas cycles for solar power applications’, Proceedings of the Institution of Mechanical Engineers 1847-1982 (vols 1-196), 193(1979), pp. 313–320. doi: 10.1243/PIME_PROC_1979_193_033_02.
Austin, N.D., Sahinidis, N.V. and Trahan, D.W. (2016) ‘Computer-aided molecular design: An introduction and review of tools, applications, and solution techniques’, Chemical Engineering Research and Design, 116, pp. 2–26. doi: 10.1016/j.cherd.2016.10.014.
Bradley, W.J. (1976) ‘Recouping the thermal-to-electric conversion loss by the use of waste heat’, in Low-grade heat: a resource in cold climates. Chalk River Nuclear Laboratories, pp. 535–558.
Carnot, S. (1824) Réflexions sur la puissance motrice du feu et sur les machines propres à développer cette puissance. Bachelier Libraire.
Cullen, J.M. and Allwood, J.M. (2010) ‘Theoretical efficiency limits for energy conversion devices’, Energy, 35(5), pp. 2059–2069. doi: 10.1016/j.energy.2010.01.024.
Gmehling, J., Constantinescu, D. and Schmid, B. (2015) ‘Group Contribution Methods for Phase Equilibrium Calculations’, Annual Review of Chemical and Biomolecular Engineering, 6, pp. 267–292. doi: 10.1146/annurev-chembioeng-061114-123424.
Grossmann, I.E. (2004) ‘Challenges in the new millennium: product discovery and design, enterprise and supply chain optimization, global life cycle assessment’, Computers & Chemical Engineering, 29(1), pp. 29–39. doi: 10.1016/j.compchemeng.2004.07.016.
Kesavan, K. and Osterle, J.F. (1982) ‘Split-Flow Nuclear Gas Turbine Cycle Using Dissociating N2O4’, in. ASME 1982 International Gas Turbine Conference and Exhibit, American Society of Mechanical Engineers Digital Collection. doi: 10.1115/82-GT-181.
Krasin, A.K. and Nesterenko, V.B. (1971) ‘Dissociating Gases: A New Class of Coolants and Working Substances for Large Power Plants’, Atomic Energy Review, 9(1), p. 177.
Lasala, S., Privat, R., Herbinet, O., Arpentinier, P., Bonalumi, D. and Jaubert, J-N. (2021) ‘Thermo-chemical engines: Unexploited high-potential energy converters’, Energy Conversion and Management, 229, 113685. doi: 10.1016/j.enconman.2020.113685.
Lighthill, M.J. (1957) ‘Dynamics of a dissociating gas. Part I: Equilibrium flow’, Journal of Fluid Mechanics, 2, pp. 1–32.
Sorokin, A. (1979) ‘Dissociating Nitrogen Dioxide (N2O4) as a working fluid in thermodynamic cycles’, Nuclear Science and Engineering, 72, pp. 330–346.
Project summary
With the aim to effectively increase the performances of power plants, refrigeration systems and heat pumps, this project proposes the use of equilibrated reactive working fluids instead of inert ones. It applies an original methodology that integrates thermodynamic and kinetic predictive tools to discover and characterise suitable reactive fluids, allowing for the quantification of the effects of reaction features on system performance and optimal architecture.
Project lead profile
Silvia Lasala, principal investigator in REACHER, is currently assistant professor at the University of Lorraine, ENSIC-LRGP (France). In 2016, she got her PhD at Politecnico di Milano (Italy) with a thesis aiming at improving the thermodynamic modelling of CO2-based streams in CO2-capture-and-storage systems. During her PhD, she also worked in designing and characterising inert working fluids for thermodynamic cycles. Then, she carried out a postdoc at LRGP (France), investigating the kinetics and thermodynamics of liquefying hydrogen and researching the possible use of inert and novel reactive working fluids in power and trigeneration plants.
Project contacts
Silvia Lasala
Laboratoire Réactions et Génie des Procédés, UMR 7274, Université de Lorraine – CNRS, Nancy Cédex, France
Email: silvia.lasala@univ-lorraine.fr
Web: www.researchgate.net/profile/Silvia-Lasala
LinkedIn: silvia-lasala-76232932
Twitter: @silvia_lasala
Funding
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101040994.
Figure legends
Figure 1: Main fluids currently used.
Figure 2: Alternative energy conversion schemes for electricity production.
Figure 3: Net work of a Brayton cycle operating with fictive fluids A2(g)D2A(g).
Figure 4: Methodology in REACHER.