Professor Scott Waddell Centre for Neural Circuits and Behaviour, University of Oxford
Millions of years ago, life began to shift from aquatic environments to dry land. This radical change of environment brought several new challenges to survival, the most obvious being a requirement to maintain hydration and avoid imminent desiccation.
Maintaining proper fluid homeostasis and osmotic balance is critical for all animal life on land. Animals regulate their water levels and osmolytes through precise physiological mechanisms that alter cellular processes and tune organismal behaviour. To absorb/maintain and excrete osmolytes, most animals possess a renal system formed by a semipermeable membrane that houses an array of transporters and channels to direct the flow of ions, organic solutes and water. These systems allow the animal to precisely regulate blood or haemolymph osmolarity to maintain optimal function. The brain senses changes in the concentration of solutes in the blood, and imbalance is ultimately translated into behaviours such as those increasing water seeking and drinking— behaviours we humans associate with our sense of thirst. In mammals, the subfornical organ (SFO) is a primary osmosensory region of the brain (Noda, 2000; Lind et al., 1984), and changes in blood osmolarity induce physiological changes in SFO principal neurons (Anderson et al., 2000). In the fruit fly Drosophila melanogaster, four cells known as the interoceptive suboesophageal neurons (ISN) neurons similarly respond to changes in haemolymph osmolarity and control both feeding and drinking behaviours (Jourjine et al., 2016).
Studies have predominantly featured neurons as the ultimate controllers of animal behaviour. However, the brain is comprised of several different cell types, and only around half of these cells are neurons. The other cells mostly consist of different types of glia, many of which are intimately associated with neurons. A complex variety of glia-neuron configurations support the structures and functions of neurons (Von Bartheld et al., 2016). Importantly, glia are a critical component of a cell layer that forms a barrier between the blood or haemolymph and the nervous system in both invertebrates and vertebrates. This anatomical position allows glia to sense changes within the internal environment and to transduce them to alter neuronal physiology. The mammalian SFO contains a collection of glial cells that express the Na(x) sodium channel critical for osmosensing by SFO principal neurons (Watanabe et al., 2006; Sakuta et al., 2019). However, our understanding of osmosensing in the brain is incomplete.
In our SCCMI project, we are using singlecell RNA sequencing to find cellular correlates of memory, motivational states and differences between individual animals. In recent work, we analysed transcriptional changes that accompany behavioural responses to thirst in brains extracted from water-sated and waterdeprived Drosophila (Park et al., 2021). We hoped to identify cell-specific changes in gene expression that might highlight brain responses involved in the control of thirstrelevant behaviours. Surprisingly, we did not observe any obvious changes in the gene expression profiles of neurons.
However, the expression of many genes were found to be regulated, either up or down, within the glia of dehydrated animals. Therefore, these initial findings supported a role for glia in sensing the fly’s circulatory environment.
To assess the functional relevance of these genes, we performed a behavioural screen to determine whether direct and independent alteration of the expression of any of the glial-regulated genes influenced water consumption. Using glial-specific expression of transgenic RNA interference constructs, we identified a gene known as astray (aay), which, when knocked down, reduced water consumption. In contrast, making more aay in glia enhanced water consumption. Testing the different types of glia revealed that aay expression is most critical in astrocytes, a glial class known to infiltrate synaptic junctions between some neurons. The aay gene encodes a phosphoserine phosphatase enzyme, which can convert phosphorylated serine into D-serine or L-serine. Since D-serine is an established co-agonist of neuronal NMDA-type (named after its selective agonist N-methyl D-aspartate) glutamate receptors, we reasoned that D-serine might mediate changes in thirst-directed behaviours by modulating the activity of glutamatergic synaptic connections in the brain.
To test this hypothesis, we fed flies with dietary serine. Strikingly, feeding D-serine but not its enantiomer L-serine increased the fly’s water consumption. Moreover, feeding D-serine could restore the drinking defect of aay-deficient flies, tightly tying loss of D-serine to a deficit in regulating thirst-relevant behaviours.
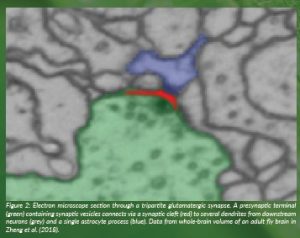
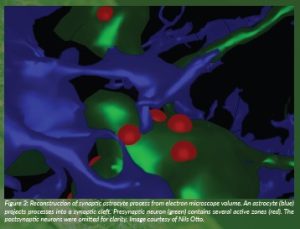
To investigate whether astrocyte glial D-serine could regulate glutamatergic synaptic connections in the fly, we reconstructed their fine morphology within an electron microscope volume of an adult fly brain (Zheng et al., 2018; Dorkenwald et al., 2022). We found that fly astrocytes extend fine processes into some synaptic junctions in a morphology that represents tripartite synapses in the mammalian brain. As their name suggests, these synaptic junctions contain pre- and postsynaptic neuronal compartments in addition to a fine process from an astrocyte (Figure 1). We also frequently found astrocyte processes that wrap around neuronal presynaptic boutons. Surprisingly, although insect brains are largely comprised of excitatory synapses that use acetylcholine as a transmitter, we found that astrocytes preferentially associate with synapses whose transmitter is glutamate.
Interestingly, D-serine acts as a neuromodulator when it binds to synaptic NMDA-type glutamate receptors. Full activation of these NMDA receptors requires binding of the neurotransmitter glutamate, D-serine and sufficient prior depolarisation of the postsynaptic neuron that they are expressed in. Therefore, synaptic connections that express NMDA receptors have a unique capacity for coincidence detection. Our SCCMI work suggests the internal state of thirst upregulates the availability of astrocytic D-serine, suggesting that certain glutamatergic synaptic connections will be most active and plastic when the animal is in this condition.
To confirm that D-serine acts through NMDA-type receptors, we demonstrated that flies carrying NMDA receptors with a genetically-modified D-serine binding site are insensitive to the thirstpromoting effects of D-serine feeding. Therefore, a clear model emerges from our work that haemolymph osmolarity changes are sensed by astrocytes that are strategically placed within the fly brain to selectively influence glutamatergic neurotransmission by supplying D-serine to NMDARs within neural circuits that promote water procuring behaviours.
Taken together, our SCCMI studies revealed a physiological context in which D-serine functions in the nervous system. We discovered that the astrocytic synthesis of D-serine is upregulated in water-deprived flies and that its release enhances the excitatory strength of glutamatergic synapses within key neuronal circuits that promote water procuring and consummatory behaviours. Given the broad infiltration of the mammalian brain by astrocytes and the abundance of glutamatergic connections expressing NMDA receptors, it seems possible that the role of D-serine in thirst will be conserved across phyla. We note that a lower D-serine serum concentration has been linked to human patients who have Schizophrenia (Hons et al., 2021) and that some of these individuals excessively drink water.
References
Anderson, J.W., Washburn, D.L.S. and Ferguson, A.V. (2000) ‘Intrinsic osmosensitivity of subfornical organ neurons’, Neuroscience, 100, pp. 539–547. doi: 10.1016/s0306-4522(00)00313-4.
Dorkenwald, S., McKellar, C.E., Macrina, T., Kemnitz, N., Lee, K., Lu, R., Wu, J., Popovych, S., Mitchell, E., Nehoran, B., Jia, Z., Bae, A., Mu, S., Ih, D., Castro, M., Ogedengbe, O., Halageri, A., Kuehner, K., Sterling, A.R., Ashwood, Z., Zung, J., Brittain, D., Collman, F., Schneider-Mizell, C., Jordan, C., Silversmith, W., Baker, C., Deutsh, D., Encarnacion-Rivera, L., Kumar, S., Burke, A., Bland, D., Gager, J., Hebditch, J., Koolman, S., Moore, M., Morejohn, S., Silverman, B., Willie, K., Willie, R., Szi-chieh, Y. and Seung, H.S. (2022) ‘Fly- Wire: online community for whole-brain connectomics’, Nature Methods, 19, pp. 119–128. doi: 10.1038/ s41592-021-01330-0.
Jourjine, N., Mullaney, B.C., Mann, K. and Scott, K. (2016) ‘Coupled sensing of hunger and thirst signals balances sugar and water consumption’, Cell, 166, pp. 855-866. doi: 10.1016/j.cell.2016.06.046.
Lind, R. W., Thunhorst, R. L., and Johnson, A. K. (1984) ‘The subfornical organ and the integration of multiple factors in thirst’, Physiology and Behavior, 32, pp. 69–74. doi: 10.1016/0031-9384(84)90072-6. Noda, M. (2006) ‘The subfornical organ, a specialized sodium channel, and the sensing of sodium levels in the brain’, The Neuroscientist, 12, pp. 80–91. doi: 10.1177/1073858405279683.
Park, A., Croset, V., Otto, N., Agarwal, D., Treiber, C.D., Meschi, E., Sims, D. and Waddell, S. (2022) ‘Gliotransmission of D-serine promotes thirst-directed behaviors in Drosophila’ bioRxiv. doi: 10.1101/2022.03.07.483255.
Sakuta, H,, Lin, C-H., Yamada, M., Kita, Y., Tokuoka, S.M., Shimizu, T. and Noda, M. (2019) ‘Nax-positive glial cells in the organum vasculosum laminae terminalis produce epoxyeicosatrienoic acids to induce water intake in response to increases in [Na+] in body fluids’, Neuroscience Research, 154, pp. 45–51. doi: 10.1016/j.neures.2019.05.006.
Von Bartheld, C. S., Bahney, J., and Herculano-Houzel, S. (2016) ‘The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting’, Journal of Comparative Neurology, 524, pp. 3865–3895. doi: 10.1002/cne.24040.
Watanabe, E., Hiyama, T.Y., Shimizu, H., Kodama, R., Hayashi, N., Miyata, S., Yanagawa, Y., Obata, K and Noda, M. (2006) ‘Sodium-level-sensitive sodium channel Na(x) is expressed in glial laminate processes in the sensory circumventricular organs’, The American Journal of Physiology – Regulatory, Integrative and Comparative Physiology, 290, R568–R576. doi: 10.1152/ajpregu.00618.2005.
Zheng, Z., Lauritzen, J.S., Perlman, E., Robinson, C.G., Nichols, M., Milkie, D., …Bock, D.D. (2018) ‘A Complete Electron Microscopy Volume of the Brain of Adult Drosophila melanogaster’, Cell, 174, pp. 730– 743. doi: 10.1016/j.cell.2018.06.019.
FIGURE LEGENDS
Figure 1: Drosophila melanogaster ready to drink from a droplet of water. Image courtesy of Suewei Lin.
Figure 2: Electron microscope section through a tripartite glutamatergic synapse. A presynaptic terminal (green) containing synaptic vesicles connects via a synaptic cleft (red) to several dendrites from downstream neurons (grey) and a single astrocyte process (blue). Data from whole-brain volume of an adult fly brain in Zheng et al. (2018).
Figure 3: Reconstruction of synaptic astrocyte process from electron microscope volume. An astrocyte (blue) projects processes into a synaptic cleft. Presynaptic neuron (green) contains several active zones (red). The postsynaptic neurons were omitted for clarity. Image courtesy of Nils Otto.
PROJECT NAME
Single-cell correlates of memory, motivation and individuality (SCCMMI)
PROJECT SUMMARY
SCCMI uses single-cell sequencing to gain a whole-brain understanding of memory and motivational states. Study of thirst revealed the primary transcriptional response in the brain to occur in glial cells. Astrocytes upregulate the expression of an enzyme required to synthesise D-serine, a coagonist of neuronal NMDA-type glutamate receptors. D-serine, in turn, facilitates neural circuits that promote water procurement behaviours.
PROJECT PARTNERS
The SCCMI project is based at the Centre for Neural Circuits and Behaviour at the University of Oxford. We collaborate with local colleagues Devika Agarwal and David Sims in Computational Genomics at the MRC Centre for Computational Biology.
PROJECT LEAD PROFILE
Scott Waddell, born in 1970 in Dunfermline (Scotland), obtained his PhD in cancer at the University of London. He switched to neuroscience for a postdoc at the Massachusetts Institute of Technology (USA). He became Group Leader at UMass Medical School (2001) and moved to Oxford in 2011. He is a Wellcome Principal Research Fellow and was awarded an ERC Advanced Grant in 2018.
PROJECT CONTACT Professor Scott Waddell Centre for Neural Circuits and Behaviour, University of Oxford, Tinsley Building, Mansfield Road, Oxford, OX1 3TA. scott.waddell@cncb.ox.ac.uk www.dpag.ox.ac.uk/team/scottwaddell @scottishwaddell
FUNDING
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 789274.