It is probably common knowledge that life expectancy in developed countries has increased exponentially since the beginning of the twentieth century. Nowadays, the number of people older than 85 years is growing faster than any other age group (Christensen et al., 2009).
Ageing is the primary risk factor for major human pathologies, including cancer, cardiovascular and neurodegenerative diseases. For this reason, the observed trend in population ageing is imposing age-associated diseases and their prevention as an important social, economic and medical topic. Although ageing research has drastically boosted over the last ten years (López-Otín et al., 2023), the molecular and cellular mechanisms of ageing still remain to be further understood. This is essential to design new approaches and therapies to extend the health span and delay or revert ageing.
One of the main hallmarks of ageing is the exhaustion of stem cells (López-Otín et al., 2023), which, over time, lose their regenerative capacity. In other words, stem cells cannot efficiently renew the cells in a tissue or repair the tissue when it is injured. Previous studies have revealed some of the changes that aged stem cells undergo, which can contribute to their poor performance. For example, mouse and human haematopoietic stem cells (HSCs) lose their cellular polarity with ageing (Florian et al., 2012), increasing symmetric cell divisions (Florian et al., 2018). This type of symmetric division results in two equal stem cells that are poor in differentiating into blood cell progenitors and that accumulate over time.
Moreover, a more recent study from our team showed that in aged HSCs, the levels and the polarity of some epigenetic marks, like the histone 4 acetylated on lysine 16 (H4K16ac), are reduced, the volume of the nucleus increases, and the number of nuclear invaginations decreases (Grigoryan et al., 2018). The loss in stem cell regenerative capacity has also been described in the skeletal muscle, a tissue that suffers an important loss in mass and function with ageing (Sousa-Victor et al., 2015). The number of skeletal muscle stem cells (MuSCs) declines around 50 per cent in aged individuals, together with a loss in their functional capacity. These observations and similar ageing effects observed in other tissues highlight the importance of stem cell decline in tissue ageing and present stem cells as an interesting target for possible rejuvenation strategies.
Several interventions for stem cell rejuvenation have been proposed, mainly based on exercise, dietary restriction and heterochronic parabiosis (shared blood circulation between a young and an old individual) (Brunet, Goodell and Rando, 2023). Although showing promising results, their effect on several types of stem cells, like HSCs, is still under debate. In the past years, we have been working on a potential stem cell rejuvenation strategy based on the inhibition of the protein Cdc42, a small RhoGTPase. This protein has been shown to be more active in aged mouse and human HSCs compared to their young counterparts and to be involved in the loss of polarity in these cells (Florian et al., 2012; Amoah et al., 2022). The pharmacological reduction of Cdc42 activity in vitro using CASIN (Cdc42 Activity Specific INhibitor) resulted in a recovery of cell polarity, H4K16ac levels, nuclear volume and shape and regenerative capacity in mouse-aged HSCs (Florian et al., 2012, 2018; Grigoryan et al., 2018). In light of these results, we now developed a systemic approach to administer CASIN treatment in vivo into aged mice by intra-peritoneal injections every 24 hours for four days. This systemic treatment increased the average and maximum lifespan of the aged mice and reduced the blood levels of several inflammatory cytokines known to increase with ageing (Florian et al., 2020). Moreover, it did not show any sign of acute or chronic toxicity. The ReSinAge project, funded by the European Research Council (ERC), aims to continue studying the effects of ageing in stem cells and tissues and the application of CASIN as a potential stem cell rejuvenating approach.
In 2022, we published the first results funded by this project in the paper “Transplanting rejuvenated blood stem cells extends lifespan of aged immunocompromised mice” (Montserrat-Vazquez et al., 2022). Here, we investigated if old HSCs and MuSCs are targeted by the CASIN treatment applied in vivo (systemically) in aged mice and if these treated stem cells alone can improve tissue regeneration, extending murine health- and lifespan. First, we studied the effects of the treatment on the health span of the mice and on their skeletal muscle tissue. We observed an improvement in the locomotor activity, endurance and strength of treated aged mice. The levels of active Cdc42 were found to be increased in aged skeletal muscle cells and to decrease with CASIN. Also, myofibre number and mass in treated aged mice were comparable to those in young mice. To challenge the tissue, we injected mice with a toxin that specifically damages the myofibres. CASIN-treated aged mice, but not control aged mice, showed an increase in the numbers and differentiation capacity of MuSCs and an improvement in skeletal muscle tissue repair and mouse fitness (endurance and strength) after the injury. Therefore, the systemic treatment with CASIN is able to target MuSCs and inhibit Cdc42 activity, improving aged MuSC regenerative capacity after injury and contributing to improved mouse fitness.
We further analysed the effects of the systemic CASIN treatment on HSCs. We confirmed that the levels of Cdc42 activity in the haematopoietic system were decreased after the treatment in vivo and that HSC polarity was significantly recovered for both Cdc42 and H4K16ac. We also analysed the localisation of HSCs in the bone marrow since it is known that the interaction of HSCs with the niche is critical for their function (Wei and Frenette, 2018). It has been described that quiescent young stem cells lie close to arterioles and the endosteum, while their aged counterparts are further from arterioles and from the endosteum (Maryanovich et al., 2018; Saçma et al., 2019; Ho and Méndez-Ferrer, 2020). Interestingly, HSCs in CASIN-treated aged mice were found closer to arterioles and to the endosteum than in young mice, indicating that the treatment affected the localisation of these stem cells and restored them to a youthful localisation. Finally, we applied single-cell RNA sequencing (scRNA-seq) to HSCs and progenitor cells of treated and untreated aged mice to study their possible gene expression changes. Although the increase of HSCs with ageing was not reduced after the treatment, we did detect changes in the expression of several genes, mainly involved in quiescence and stress response. CASIN also partially restored the gene expression heterogeneity that was detected to be lost with ageing. Finally, an analysis of the transcriptional connectivity between the different cell types revealed a huge connectivity loss with ageing that CASIN partially reverted.
Considering this ‘youthful’ HSC phenotype observed after the treatment, we performed transplantation assays with the systemically treated aged HSCs transplanted into lethally irradiated mice to analyse their regenerative capacity. HSCs obtained from CASIN-treated aged mice were better at reconstituting the recipients’ haematopoietic system compared to those from control aged mice. Also, the frequency of B cells in the peripheral blood was higher after the transplant with aged CASIN-treated cells, indicating an improvement in the B-lymphoid regenerative capacity of aged HSCs after the systemic CASIN treatment. We also transplanted the treated aged HSCs into aged immunocompromised mice to more broadly test the effect on aged recipients’ health span and lifespan. Noteworthy, aged mice that received HSC from aged CASIN-treated mice presented with extended median and maximum lifespan by ~25 per cent and ~34 per cent, respectively, compared to the aged mice transplanted with the control aged HSCs or a group of aged mice that didn’t receive any transplanted cell. Therefore, CASIN-rejuvenated HSCs are sufficient to extend the lifespan of aged recipient mice upon transplantation.
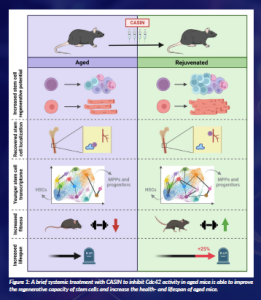
Altogether, we have shown that a brief systemic treatment with CASIN to inhibit Cdc42 activity in aged mice is able to improve the regenerative capacity of stem cells and increase the health- and lifespan of aged mice (Figure 1). These findings support CASIN treatment as a promising strategy for aged stem cell rejuvenation and extend to the possibility of targeting endogenous aged stem cells as a promising strategy to extend health span and lifespan, with an interesting translational potential into humans. As part of the ReSinAge project, we are continuing to study possible applications of Cdc42 activity inhibition in vivo for translational applications in the context also of tissue regeneration after, for example, chemotherapy.
Watch: Is it possible to extend lifespan? The role of stem cells.
Disclosure
MC Florian is part of the advisory board of MoglingBio.
References
Amoah, A. Keller, A., Emini, R., Hoenicka, M., Liebold., A., Vollmer, A., Eiwen, K., Soller, K., Sakk, V., Zheng, Y., Florian, M.C. and Geiger, H. (2022) ‘Aging of human hematopoietic stem cells is linked to changes in Cdc42 activity’, Haematologica, 107(2), pp. 393–402. doi: 10.3324/haematol.2020.269670.
Brunet, A., Goodell, M.A. and Rando, T.A. (2023) ‘Ageing and rejuvenation of tissue stem cells and their niches’, Nature Reviews Molecular Cell Biology, 24(1), pp. 45–62. doi: 10.1038/s41580-022-00510-w.
Christensen, K., Doblhammer, G., Rau, R. And Vaupel, J.W. (2009) ‘Ageing populations: the challenges ahead’, Lancet, 374(9696), pp. 1196–1208. doi: 10.1016/S0140-6736(09)61460-4.
Florian, M.C., Dörr, K., Niebel, A., Daria, D., Schrezenmeier, H., Rojewski, M., Filippi, M-D., Hasenberg, A., Gunzer, M., Scharffetter-Kochanek, K., Zheng, Y. and Geiger, H. (2012) ‘Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation’, Cell Stem Cell, 10, pp. 520–530. doi: 10.1016/j.stem.2012.04.007.
Florian, M.C., Klose, M., Sacma, M., Jablanovic, J., Knudson, L., Nattamai, K.J., Marka, G., Vollmer, A., Soller, K., Sakk, V., Cabezas-Wallscheid, N., Zheng, Y., Mulaw, M.A., Glauche, I. And Geiger, H. (2018) ‘Aging alters the epigenetic asymmetry of HSC division’, PLoS Biology, 16(9), e2003389. doi: 10.1371/journal.pbio.2003389.
Florian, M.C., Leins, H., Gobs, M., Han, Y., Marka, G., Soller, K., Vollmer, A., Sakk, V., Nattamai, K.J., Rayes, A., Zhao, X., Setchell, K., Mulaw, M., Wagner, W., Zheng, Y., and Geiger, H. (2020) ‘Inhibition of Cdc42 activity extends lifespan and decreases circulating inflammatory cytokines in aged female C57BL/6 mice’, Aging Cell, 00, p. e13208. doi: 10.1111/acel.13208.
Grigoryan, A. (2018) ‘LaminA/C regulates epigenetic and chromatin architecture changes upon aging of hematopoietic stem cells’, Genome Biology, 19(189), pp. 1–21. doi: 10.1186/s13059-018-1557-3.
Ho, Y.H. and Méndez-Ferrer, S. (2020) ‘Microenvironmental contributions to hematopoietic stem cell aging’, Haematologica, 105(1), pp. 38–46. doi: 10.3324/haematol.2018.211334.
López-Otín, C., Blasco, M.A., Partridge, L., Serrano, M. and Kroemer, G. (2023) ‘Hallmarks of aging: An expanding universe’, Cell, 186, pp. 243–278. doi: 10.1016/j.cell.2022.11.001.
Maryanovich, M., Zahalka, A.H., Pierce, H., Pinho, S., Nakahara, F., Asada, N., Wei, Q., Wang, X., Ciero, P., Xu, J., Leftin, A. and Frenetter, P.S. (2018) ‘Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche’, Nature Medicine, 24(6), pp. 782–791. doi: 10.1038/s41591-018-0030-x.
Montserrat-Vazquez, S., Ali, N.J., Matteini, F., Lozano, J., Zhaowei, T., Mejia-Ramirez, E., Marka, G., Vollber, A., Soller, K., Saçma, M., Sakk, V., Mularoni, L., Mallm, J.P., Plass, M., Zheng, Y., Geiger, H. And Florian, M.C. (2022) ‘Transplanting rejuvenated blood stem cells extends lifespan of aged immunocompromised mice’, npj Regenerative Medicine, 7(78). doi: 10.1038/s41536-022-00275-y.
Saçma, M., Pospiech, J., Bogeska, R., de Back, W., Mallm, J-P., Sakk, V., Soller, K., Marka, G., Vollmer, A., Karns, R., Cabezas-Wallscheid, N., Trumpp, A., Méndez-Ferrer, S., Milsom, M.D., Mulaw, M.A., Geiger, H. and Florian, M.C. (2019) ‘Haematopoietic stem cells in perisinusoidal niches are protected from ageing’, Nature Cell Biology, 21(11), pp. 1309–1320. doi: 10.1038/s41556-019-0418-y.
Sousa-Victor, P., García-Prat, L., Serrano, A.L., Perdiguero, E. And Muñoz-Cánoves, P. (2015) ‘Muscle stem cell aging: Regulation and rejuvenation’, Trends in Endocrinology and Metabolism, 26(6), pp. 287–296. doi: 10.1016/j.tem.2015.03.006.
Wei, Q. and Frenette, P.S. (2018) ‘Niches for Hematopoietic Stem Cells and Their Progeny’, Immunity, 48(4), pp. 632–648. doi: 10.1016/j.immuni.2018.03.024.
Project name
ReSinAge (ERC consolidator)
Project summary
Cancer is a disease of the elderly, and chemotherapy remains the mainstay of treatment. Unfortunately, older patients are highly susceptible to specific toxicities of chemotherapy, like myelosuppression and life-threatening neutropenia. ReSinAge explores the possibility of improving the regenerative capacity of the aged stem cell niche as an unprecedented, innovative strategy to improve the haematopoietic recovery and increase the survival after chemotherapy in the elderly. More broadly, we aim to provide proof-of-concept evidence that increasing the regenerative potential of endogenous aged somatic stem cells represents an important strategy for rejuvenating tissues and improving health- and lifespan in the elderly.
Project lead profile
Dr Florian started her independent career focusing on stem cell ageing as Emmy Noether (DFG) junior group leader at the Institute of Molecular Medicine, University of Ulm (Ulm, Germany) in 2016. At the end of 2018, she was appointed by the Programme for the Clinical Translation of Regenerative Medicine in Catalonia (P-CMRC) and the Programme of Regenerative Medicine at IDIBELL (Barcelona, Spain). In 2023, she became ICREA Research Professor (ICREA).
Dr Florian’s lab focuses on understanding cellular and molecular mechanisms of somatic stem cell ageing, supporting the development of new therapeutic strategies to preserve the regenerative capacity of stem cells over time and to limit or prevent the development of age-related disorders and extend healthspan and lifespan.
Project contacts
Dr M. Carolina Florian
ICREA Research Professor (Group Leader)
Stem Cell Ageing, Regenerative Medicine,
IDIBELL
https://orcid.org/0000-0002-5791-1310
https://p-cmrc.cat/research/florian-group
linkedin.com/in/m-carolina-florian-666bb783
Figure legends
Figure 1: A brief systemic treatment with CASIN to inhibit Cdc42 activity in aged mice is able to improve the regenerative capacity of stem cells and increase the health- and lifespan of aged mice.