Most people know someone suffering from cardiovascular disease. There are 15 million people in Europe currently living with heart failure. Not only does the condition lead to a lasting and significant impact on physical health, but it also affects overall well-being, manifesting in issues such as anxiety or mood swings.
Unlike other cardiovascular diseases, the prevalence of heart failure continues to rise. While it is clear that solutions are required, it is especially difficult to find new drugs or devices for this disease. Despite significant investments in healthcare, the number of new drug approvals is not increasing. Why? Because of barriers such as the high cost of R&D, regulatory complexity and difficulty in commercialising new products.
Digital transformation
The power of digital technology and data exchange is known to support innovation: artificial intelligence (AI), high-performance computing, cloud computing, and the internet of things continue to impact our everyday lives. Our health data is no exception. It can significantly contribute to better disease prevention, early diagnosis, and the development of a personalised service.
In-silico methods are computational predictions—in-silico referring to the silicon in our computers. These in-silico methods can make fast predictions for large sets of compounds in a high-throughput mode.
In-silico computational approaches can be used to predict complex clinical scenarios.
Health care and digital innovation in Europe
The European Commission (EC) supports using these new technologies and health data in the digital transformation of health and care. Hence the EC is funding SimCardioTest to demonstrate the feasibility, efficacy and benefits of in-silico trials for disease. Specifically, SimCardioTest aims to provide insight into designing new predictive tools in cardiac pathologies and accelerate the uptake of computer simulations for testing medicines and medical devices.
SimCardioTest
SimCardioTest has been funded for four years (2020-24) to create a secure, standardised cloud-based platform that allows seamless in-silico trial runs. Its goal is to win the trust of patients, scientists, regulators, physicians and other healthcare professionals and promote innovation in Europe and elsewhere.
The project brings together outstanding expertise in European computer modelling, simulation, and medical devices. There are ten project partners led by the Institut National de Recherche en Informatique et Automatique, France. The other partners are Université de Bordeaux (UBx), France; Universitat Pompeu Fabra (UPF) and Universitat Politècnica de València, (UPV), Spain; Simula Research laboratory AS (SRL), Norway; InSilicoTrials Technologies SPA (IST), Italy; SORIN CRM SAS (MPC) and ExactCure (EXC), France; Boston Scientific Scimed Inc (BSC), United States; and the Virtual Physiological Human Institute for Integrative Biomedical Research VZW (VPHi), Belgium.
The partners are supported by an advisory board composed of the European Agency for Medicine, the Food and Drug Administration in the United States, the University of Lyon, Dassault Systèmes, the pharmaceutical company Roche, and Auckland Bioengineering Institute in New Zealand.
SimCardioTest will demonstrate the benefit of in-silico trials testing three use cases in cardiology.
How is a simulation obtained?
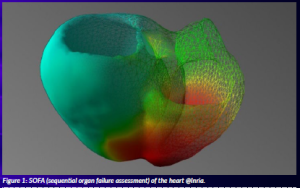
New predictive tools can now be created thanks to access to high-performance computing infrastructures (HPC), patient-specific information, and big data analysis based upon AI. Computational simulation is based on the deep analysis of medical images such as 3D computed tomography (CT) scans, echography and magnetic resonance images. These images are processed with AI tools to create a 3D model that is specific for each patient (Figure 1).
The process enables the exact simulation of some characteristics of a patient’s heart, with their specific morphology, electrical currents, and can also be related to their age, gender, and weight.
These computational simulations are powerful tools developed for personalised, adapted medicine.
Three use cases as demonstrators in cardiac pathologies
To test drugs and devices, specific computational models will be created for cardiac pathologies such as ischaemia, atrial fibrillation, and heart failure.
1. Pacing devices used in cardiac arrhythmias associated with heart failure
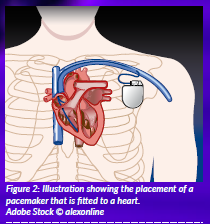
With electrical impulses regulated by specific heart cells, the heart beats synchronously. A pacemaker or defibrillator (a small battery-powered device) will help maintain a normal heart rhythm and effective heart contractions when your heart beats asynchronously (arrhythmia, fibrillation). These devices are placed under the skin and linked by a flexible wire to small electrodes anchored in the heart muscle (Figure 2). The electrodes deliver electrical impulses to the heart that are generated and controlled by the pacemaker.
SimCardioTest’s objective is to create computational models of the navigation of the lead from its insertion point towards its final location in the heart and evaluate its pacing properties at this location. In the models, the risks of perforation and rupture of the heart from the fatigue of leads will be evaluated, as well as the threshold of energy required for the stimulation of a heartbeat for typical designs of the device. This work will impact the future design of pacemakers or defibrillation devices.
Scientists will run computer simulations and validate their numerical models with observations from realistic physical phantoms of the heart and the vascular tree of interest created using 3D printing and soft robotics. They will also acquire experimental data of the heart’s electrical activation with an optical fluorescence technique.
2. Left atrial appendage occluder to reduce stroke risk.
The left atrial appendage (LAA) is a small vesicle located in the muscular wall of the left atrium. In atrial fibrillation, blood can form clots in this vesicle, and when blood clots are pumped out of the heart, they can cause a stroke. To overcome the risks, left atrial appendage occluders (LAAO) have been designed to close the LAA in patients with atrial fibrillation to prevent the formation of clots.
LAA occlusion is common in patients who are intolerant of anticoagulant therapy or who have bleeding risks associated with the use of such therapy.
3. Left atrial appendage occlusion reduces the risk of stroke in atrial fibrillation.
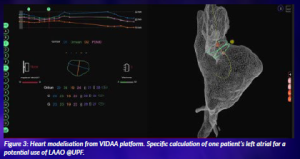
SimCardioTest uses 3D CT images and echocardiography to characterise the left atrial morphology (Figure 3) and simulate its haemodynamics to determine the best configuration (i.e. one that is less likely to cause thrombus formation) to implant occluder devices. This will allow us to model the entire treatment cycle, including thrombus formation, drug treatment models, and prediction of treatment response.
Drugs safety, efficacy and cardiotoxicity
The third study will assess drug safety and efficacy in personalised and detailed models. The model will allow for the measurement of the impact of different drug dosages based on patient characteristics. This model will be built at the cellular level and organ level. This is a significant advance in computational simulation.
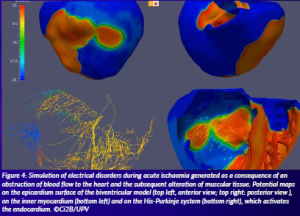
These simulations will take into account both healthy and pathological conditions such as ischaemia (Figure 4), heart failure or atrial fibrillation, as well other patient characteristics like age and gender. A smartphone could be used to easily track the outcome.
In-silico trials
On a cloud-based platform that is pre-standardised, models for different pathologies can be tested. To quantify the realism of the in-silico results, simulations will be performed that correspond to real measurements. The richness of the generated data could lead to the development of new indices to evaluate medical device design and drug efficacy/toxicity.
These simulations will be thoroughly verified as the results can support the certification process. This will be done in close collaboration with regulatory by organisations.
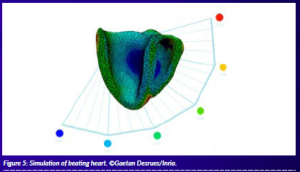
In-silico trials can be used to develop drugs and devices, reduce costs and time-to-market, and gain the trust and support of patients, scientists, regulators, doctors, and physicians. The trials will also prove that this approach can replace some of the invasive aspects of clinical trials, reduce the number of animal tests and provide new biomarkers to improve the accuracy in prediction from clinical trials (Figure 5).
Conclusion
SimCardioTest will demonstrate the effectiveness of a cloud-based platform for new device and drug testing by combining computer modelling and simulation with AI, using patient-specific data and HPC. This way, SimCardioTest aims to revolutionise cardiac research and facilitate the translation of therapies from the laboratory to the hospital and the patients.
Figure legends
Figure 1: SOFA (sequential organ failure assessment) of the heart @Inria.
Figure 2: Illustration showing the placement of a pacemaker that is fitted to a heart. Adobe Stock © alexonline
Figure 3: Heart modelisation from VIDAA platform. Specific calculation of one patient’s left atrial for a potential use of LAAO @UPF.
Figure 4: Simulation of electrical disorders during acute ischaemia generated as a consequence of an obstruction of blood flow to the heart and the subsequent alteration of muscular tissue. Potential maps on the epicardium surface of the biventricular model (top left, anterior view; top right: posterior view ), on the inner myocardium (bottom left) and on the His-Purkinje system (bottom right), which activates the endocardium. ©Ci2B/UPV.
Figure 5: Simulation of beating heart. ©Gaetan Desrues/Inria.
Article summary
PROJECT SUMMARY Cardiovascular diseases affect 15 million people in Europe, and digital solutions are now seen as very useful tools in the search for new drugs and medical devices. SimCardioTest is a 4-year project funded by the European Commission that aims to develop credible computer modelling and simulation approaches on a cloud-based platform for testing cardiac drugs and devices in silico.
PROJECT LEAD SimCardioTest is led by Inria, the French national research institute for the digital sciences. A world-class research and technological innovation organisation, Inria develop and support scientific and entrepreneurial projects that create value in France and Europe. Dr Maxime Sermesant, Head of Computational Cardiology at Inria Epione and Chair of AI and Biophysics at 3IA Côte d’Azur, ensures the scientific coordination.
PROJECT PARTNERS SimCardioTest brings together leading experts in the field of cardiac simulation, drug effect, medical devices and regulatory process. It includes large companies (Microport – CRM and Boston Scientific), SMEs (ExactCure and InSilicoTrials), research organisations (Inria and Simula), universities (University of Bordeaux, University Pompeu Fabra, Polytechnic University of Valencia) and an international non-profit organisation (The Virtual Physiological Human Institute).
CONTACT DETAILS Michèle Barbier Inria Sophia Antipolis-Méditerranée, 2004 Route des Lucioles, 06902 Valbonne, France +336 3307 9899 michele.barbier@inria.fr https://www.simcardiotest.eu @SimCardioTest /SimCardioTest
FUNDING This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No.101016496.