Alexander Bartelt
Ludwig-Maximilians-Universität München, Germany
Leonardo Matta
Helmholtz Center Munich, Germany
Obesity: a big challenge for our society
The alarming increase in obesity in our society is a multifaceted issue that demands targeted public strategies and new medical therapies. Numerous factors facilitate this development, reflecting substantial changes in our way of life over the past few decades. Diets have transitioned from natural whole foods to ultra-processed, energy-dense, high-fat and high-sugar alternatives, resulting in a positive energy balance and weight gain across children and adults.
The World Health Organization (2022) reports that currently, obesity affects approximately 1 billion people worldwide. The World Obesity Atlas (2023) describes how obesity has a profound economic impact on the world, estimated at around four trillion dollars per year for obesity-linked comorbidities.
Another important concern is sedentary lifestyles, further exacerbating the weight gain problem, as physical inactivity is linked to accelerated metabolic disease. Over millennia, humans evolved to manage their energy expenditure judiciously to survive in environments where food was often scarce and, unlike today, was not readily available. In the modern world, this genetic predisposition becomes a significant obstacle, as our bodies are primed to store energy efficiently, often without wasting a single calorie. In addition, our brain is wired to seek out nutritious foods and is very resistant to letting the body slip into a negative energy balance.
This collision of environmental and genetic factors evidently makes losing weight challenging. However, physical exercise has been proven to be a vital lifestyle intervention in addressing metabolic problems. Even in the absence of meaningful weight loss, exercise and physical activity have a profound impact on cardiometabolic health.
Our research focuses on skeletal muscle tissue, aiming to unravel its molecular role as a key player in mitigating obesity-induced cardiometabolic disorders.
Physical activity and exercise for cardiometabolic health
It is well known that physical exercise has a beneficial impact on reducing obesity-linked comorbidities such as diabetes and cardiovascular disorders. Nonetheless, adherence to exercise as a lifestyle intervention remains a formidable challenge for the patient—and sometimes even for the rather healthy layperson as well. For leveraging the beneficial effects of exercise, it is imperative to develop multifaceted strategies combining education about dietary strategies and physical activity behaviour for the individual on the one hand and, on the other hand, public health measures to support healthier lifestyles. These changes must be disseminated and applied in evidence-based policies and interventions that empower individuals to make healthier choices in their day-to-day lives.
Since 2000, several initiatives and non-governmental organisations have promoted physical exercise as an important intervention against obesity. The scientific community has been developing one of the most known movements to fight against sedentarism and obesity, called ‘exercise is medicine’. WHO recommends 150–300 minutes of moderate exercise per week to reduce detrimental health risks caused by sedentary behaviour (Bull et al., 2020).
However, physical activity cannot combat obesity alone when dietary behaviour remains unchanged. Nevertheless, it is encouraging that muscle cells exhibit high adaptability, quickly adjusting to increased demands when used more frequently. Thus, maintaining the health of our muscle tissue and its constituent cells, known as myocytes, is paramount.
Physical exercise can be broadly categorised into endurance or strength challenges and is characterised by a structured and planned sequence of body movements with appropriate control of the intensity (load) and volume (quantity of exercises and session duration). As such, one could view exercise sessions as a stressful intervention for our bodies, which induces our cells to adapt to this controlled ‘dosage’ of stress induced by skeletal muscle triggering (Louzada et al., 2020). In the end, exercise has a hormetic effect, as the molecular response to acute, beneficial stress induces physiologic adaptations such as enhanced metabolic capacity, reduced fatigue, muscle growth, and increased strength and/or endurance in the long run.
Energetic muscle cells burn calories and talk to the body
At the cellular level, muscle tissue is largely made up of skeletal muscle cells, the so-called myocytes. Skeletal muscle tissue represents approximately 40 per cent of the body mass in a lean individual and fulfils several functions for movement and posture. In addition, muscle serves as an endocrine organ, exerting an important role in coordinating whole-body metabolism at rest and especially during physical activity and exercise (Chow et al., 2022). Incorporating a small exercise routine into our daily schedules or generally increasing physical activity levels substantially impacts muscle physiology.
It is well established that exercise induces muscle damage. In physiological conditions, myocytes are regenerated by the activation of satellite cells, which are stem cells that differentiate into myoblasts and fuse to form new muscle fibres, also referred to as myogenesis. This process is essential for repairing any damage induced by the exercise and improving muscle capacity due to remodelling towards enhanced myocytes. Healthy skeletal muscle is crucial for an anti-inflammatory secretion profile, and these myokines play significant roles in maintaining insulin sensitivity and protecting against metabolic dysfunction.
In conclusion, skeletal muscle function is important not only for moving around but also serves as an integral endocrine player in our metabolism and exerts far-reaching effects on our overall metabolic health and wellbeing. In response to training-induced stress, muscle cells adapt by fine-tuning metabolic pathways, cellular signalling, gene expression, androtein breakdown and synthesis (Atherton, Phillips and Wilkinson, 2015). To a certain extent, exercise induces muscle damage and the need to recycle parts of the cell. Aside from protein synthesis, muscle cells also need an effective system for recycling proteins that are damaged in response to acute exercise. The increased protein turnover and breakdown are important mechanisms for maintaining cellular quality control, especially in terms of muscle protein synthesis, remodelling and regeneration. Understanding the molecular regulators of protein breakdown, synthesis and clearance is crucial for muscle tissue regeneration and function. It could open the path to new therapies for enhanced muscle function or combatting metabolic diseases.
Protein degradation for protein recycling
For an optimal response to short-term and long-term exercise, myocytes need to maintain a delicate balance between protein synthesis, folding and degradation, referred to as proteostasis. In our ERC project PROTEOFIT, we aim to understand the molecular regulatory mechanisms governing protein degradation in muscle, a pivotal yet frequently underappreciated cellular quality control process.
While all cells of our body have mechanisms for the clearance of metabolites and proteins, the primary degradation pathway operating within skeletal muscle cells is the ubiquitin-proteasome system (UPS). The proteasome is a vital cellular component responsible for upholding quality control of proteins within the cell. The removal of damaged or obsolete proteins is fundamental to avoid perturbations of cell homeostasis and function.
Interestingly, in muscle tissue, this process is triggered by both beneficial exercise and harmful obesity, presenting a somewhat paradoxical situation.
Our research is dedicated to elucidating the fundamental significance of proteasomal protein breakdown in the modulation of proteostasis and protein metabolism in resting conditions, during the adaptive response to exercise, and in conditions of metabolic disease. It is anticipated that a large part of the relevance of the proteasome is protein quality control. However, much less is known about its role in breaking down proteins into their constituent parts, resulting in increased availability of amino acids, which can be used to synthesise new proteins. Considering that some amino acids are essential in that they cannot be synthesised but must be taken up with the diet, muscle proteins represent a large reservoir of amino acids.
We and others have recently investigated the gene switch transcription factor NFE2, like bZIP transcription factor 1 (NFE2L1), which directly modulates UPS function by regulating proteasomal gene expression (Bartelt et al., 2018). NFE2L1 exhibits complex biology. Normally, it’s degraded by the proteasome and is scarcely present in the cell. However, when the proteasome is overwhelmed or chemically inhibited, NFE2L1 avoids degradation. This allows it to fine-tune protein breakdown by boosting the expression of proteasome genes. As a result, it adjusts the rates of protein degradation and recycling. Notably, while NFE2L1 is highly expressed in muscle tissue, its specific functions in myocytes are still largely unexplored.
Main results (and curious findings)
During the ERC PROTEOFIT project, our research has demonstrated that NFE2L1 plays a pivotal role as a regulator of proteasome genes in isolated muscle cells and transgenic mouse models (Lemmer et al., 2023). In a controlled cell culture environment designed for studying metabolism in a more isolated context, the absence of NFE2L1 results in the accumulation of ubiquitinated proteins (referred to as protein waste) due to impaired degradation by the proteasome. Using complex proteomic strategies, we charted the proteins targeted for degradation and discovered new signalling key nodes and new metabolic players in muscle function and regeneration. While these findings might not come as a surprise, NFE2L1-mediated proteasome function impacts skeletal muscle on multiple levels, depending on the condition.
Using our entire toolbox of transgenic mouse models, we found that NFE2L1 impacts muscle development, regeneration and the response to a high-fat diet. Loss of NFE2L1 in mice is associated with weaker muscles and lower physical capacity. Curiously, they display an enhanced energy metabolism and are more protected against high-fat diet-induced obesity (Lemmer et al., 2023). We also observed that, in response to exercise, NFE2L1 is crucial for skeletal muscle recovery.
Exercise increases protein damage, leading to protein ubiquitination for the removal of damaged proteins and the generation of amino acids for protein synthesis and muscle regeneration. The absence of NFE2L1 impairs UPS function, resulting in elevated levels of dysfunctional and damaged proteins within the cell and leading to inflammation in skeletal muscle cells, which hinders the beneficial adaptations induced by exercise.
Our data points to the fact that NFE2L1 is essential for unlocking the complete capabilities of muscle cells. We expect that our work provides a framework for understanding proteostasis in muscle and finally untangling the curious regulation of UPS on obesity and exercise.
In summary, our ERC project PROTEOFIT allows us to address an unmet need for understanding the molecular underpinnings of energy metabolism in a field of increasing significance: sedentary lifestyles and the emergence of obesity-related conditions in Europe and beyond.
References
Atherton, P.J., Phillips, B.E. and Wilkinson, D.J. (2015) ‘Exercise and Regulation of Protein Metabolism’, Progress in Molecular Biology and Translational Science, 135, pp. 75–98. doi: 10.1016/ BS.PMBTS.2015.06.015.
Bartelt, A., Widenmaier, S.B., Schlein, C., Johann, Kornelia, J., Goncalves, R.L.S., Eguchi, K., Rischer, A.W., Paralakgül, G., Snyder, N.A., Nguyen, T.B., Bruns, O.T. Franke, D., Bawendi, M.G., Lynes, M.D., Leiria, L.O., Tseng, Y-H., Inouye, K.E., Arruda, A.P. and Hotamisligil, G.S. (2018) ‘Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity’, Nature Medicine, 24(3), pp. 292–303. doi: 10.1038/nm.4481.
Bull, F.C., Al-Ansari, S.S., Biddle, S., Borodulin, K., Buman, M.P., Cardon, G., Carty, C., Chaput, J.P., Chastin, S., Chou, R., Dempsey, P.C., DiPietro, L., Ekelund, U., Firth, J., Friedenreich, C.M., Garcia, L., Gichu, M., Jago, R., Katzmarzyk, P.T., Lambert, E., Leitzmann, M., Milton, K., Ortega, F.B., Ranasinghe, C., Stamatakis, E., Tiedemann, A., Troiano, R.P., van der Ploeg, H.P., Wari, V. And Willumsen, J. F. (2020). ‘World Health Organization 2020 guidelines on physical activity and sedentary behaviour’, British Journal of Sports Medicine, 54(24), pp. 1451–1462. doi: 10.1136/bjsports-2020-102955.
Chow, L.S., Gerszten, R.E., Taylor, J.M., Pedersen, B.K., van Praag, H., Trappe, S., Febbraio, M.A., Galis, Z.S., Gao, Y., Haus, J.M., Lanza, I.R., Lavie, C.J., Lee, C.H., Lucia, A., Moro, C., Pandey, A., Robbins, J.M., Stanford, K.I., Thackray, A.E., Villeda, S., Villeda, S., Watt, M.J., Xia, A., Zierath, J.R., Goodpaster, B.H. and Snyder, M.P. (2022) ‘Exerkines in health, resilience and disease’, Nature Reviews Endocrinology, 18(5), pp. 273–289. doi: 10.1038/S41574-022-00641-2.
Lemmer, I.L., Hass, D.T., Willemsen, N., Kotschi, S., Toksöz, I., Gjika, E., Khani, S., Rohm, M., Diercksen, N., Nguyen, P.B.H, Menden, M.P., Egu, D.T., Waschke, J., Larsen, S., Ma, T., Gerhart-Hines, Z., Herzig, S., Dyar, K., Krahmer, N and Bartelt, A., (2023) ‘Nfe2l1-mediated proteasome function controls muscle energy metabolism in obesity’, bioRxiv, p. 2023.04.20.537611. doi: 10.1101/2023.04.20.537611.
Louzada, R.A., Bouviere, J., Matta, L.P., Werneck-de-Castro, J.P., Dupuy, C., Carvalho, D.P. and Fortunato, R.S. (2020) ‘Redox Signaling in Widespread Health Benefits of Exercise’, Antioxidants & Redox Signaling [Preprint]. doi: 10.1089/ars.2019.7949.
WHO (2022) World Obesity Day 2022 – Accelerating action to stop obesity. Available at: https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity. (Accessed: 16 October 2023).
Project summary
Project name
PROTEOFIT – Adapting Protein Fate for Muscle Function and Fitness
Project summary
Physical activity and exercise have beneficial effects on overall fitness and health. However, exercise-induced increases in metabolism require specific molecular adaptation of the muscle cells. This project aims to understand the molecular processes in the muscle during the adaptation to cold, exercise and obesity, thereby defining novel mechanisms of protein homeostasis with regard to the gene switch Nfe2l1.
Project lead
Alexander Bartelt is the Professor of Cardiovascular Metabolism at the Institute for Cardiovascular Prevention, Ludwig-Maximilians-University Munich. He received his diploma in biochemistry in 2007 and his PhD in 2010 from the University of Hamburg, Germany, with honours, after his postdoctoral training at Harvard University, USA. The Bartelt lab is dedicated to understanding the basic principles of metabolic adaptation as well as the molecular pathology of cardiometabolic diseases.
Over the years, Dr Bartelt’s contributions have been recognised by national and international awards and distinctions.
Project partners
This project is based at the Institute for Cardiovascular Prevention (IPEK) at the LMU Munich in collaboration with the Helmholtz Diabetes Center Munich.
Contact details
Univ.-Prof. Dr Alexander Bartelt
alexander.bartelt@med.unimuenchen.de
Dr Leonardo Matta
leonardo.matta@helmholtzmuenchen.de
Funding
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 852742.
Image legends
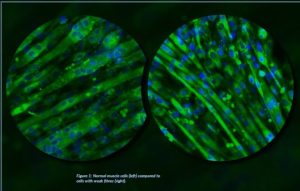
Figure 1: Normal muscle cells (left) compared to cells with weak fibres (right).
Figure 2: Dr Matta using exercise equipment to study metabolism in laboratory transgenic mouse models.
Figure 3: Dr Matta and Prof. Bartelt discussing new ways to enhance muscle function by molecular therapy.