Endometriosis is a common heterogeneous gynaecological disorder
Endometriosis is a common gynaecological disease defined by the presence of lesions of functional endometrial tissue outside the uterine cavity (ectopic endometrium). It affects approximately 10 per cent of women in their reproductive years (from puberty to menopause).
The most common symptoms are pelvic pain (during the menses and chronically) and pain during intercourse (Chapron et al., 2019). Usually, the intensity of these pain symptoms is high. Other symptoms include painful bowel movements/ constipation/diarrhoea, painful urination, fatigue, depression or anxiety, abdominal bloating and nausea. In some cases, endometriosis can be asymptomatic. So, the manifestation of the disease is quite variable across patients, participating in the delay of its diagnosis. Furthermore, infertility, which can be seen as a silent symptom (unknown until the patient tries to conceive), is associated with endometriosis in around 40 per cent of patients. Endometriosis is also associated with an increased risk of miscarriage and pregnancy complications (Leone Roberti Maggiore et al., 2016).
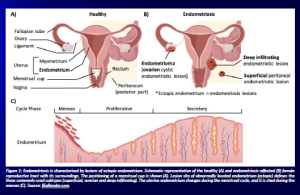
Endometriosis patients can be classified into three subgroups according to the lesion localisation and infiltration: superficial peritoneal lesions, ovarian endometriosis (also called endometrioma) and deep infiltrating endometriosis (Chapron et al., 2019).
So endometriosis is a major health issue that strongly affects the quality of life of patients and is an economic burden, with a cost estimated at ≈10 k€ per year per patient (Simeons et al., 2012).
Endometriosis diagnosis
As the symptoms are not very specific (and sometimes wrongly dismissed), the diagnosis of endometriosis is difficult and is usually considered definitive only after a lesion histological analysis, requiring an invasive procedure. However, the current guidelines (from several national and international societies) recommend the use of hormonal therapy as a first line of treatment in suspected endometriosis cases to avoid unnecessary invasive procedures if the medical treatment is efficient in reducing the pain symptoms (Kalaitzopoulos et al., 2021). Imaging is also a proposed tool in diagnosing endometriosis, i.e. transvaginal ultrasound (TVUS) and magnetic resonance imaging (MRI). Depending on the lesion localisation and the interpreter experience, the detection rate with these methods is highly variable. Therefore, using imaging for endometriosis diagnosis has several limitations: the cost of these technologies, the false negative rate that is not negligible (5 to 20 per cent) and their effectiveness that is highly dependent on the image interpretation, an expertise rarely available in a primary care setting. So, one of the current high-stakes goals of endometriosis patient care is the development of reliable methods for early non-invasive diagnosis. Despite numerous efforts, in a variety of tissues, but mainly in peripheral blood or semi-invasively in endometrium or peritoneal fluid (Brulport et al., 2024), no biomarkers or a combination of those (including imaging data) was found and validated with sufficient sensitivity and specificity for use in clinical practice (Nisenblat et al., 2016a–b; Gupta et al., 2016). As a result of these aspects, there is a delayed diagnosis, estimated to be eight years (Ghai et al., 2020).
Endometriosis treatment
The current treatments for endometriosis are based on medical and surgical approaches for pain management and assisted reproductive technologies (ART) (and/or surgery) for infertility. There is still a lot of dissensus for endometriosis treatment (Kalaitzopoulos et al., 2021) and a global lack of long-term efficacy of the available options.
The medical treatment (Barbara et al., 2021; Barra et al., 2019; Becker et al., 2017; Donnez and Dolmans, 2021) basically consists of inducing amenorrhea (to suppress the shedding of the endometriotic lesions) using hormonal treatments, in combination with pain meds if necessary. As such, it is symptomatic and not curative, and the beneficial effect is usually lost upon treatment cessation. Usually proposed as first-line therapies are the combined oral contraceptives (oestrogen-progestins) and progestins. Their efficacy on dysmenorrhoea is usually higher than on non-menstrual pelvic pain. Gonadotropin-releasing hormone—GnRH—agonists and antagonists are proposed as second-line treatments. They were shown to be effective on menstrual and non-menstrual pelvic pain. Concerning the response to these medical treatments, 15–30 per cent of patients do not respond to these options. These medical options have shown no benefit for the fertility of endometriosis patients.
The surgical treatment (Kalaitzopoulos et al., 2021; Singh et al., 2020) is a potentially curative option. The recommendation for surgery is a lack of pain relief (due to medical treatment inefficacy or intolerance). Surgical excision of all the lesions by laparoscopy is recommended in an expert centre as lesions may involve several other organs from the pelvic cavity (bowel, urinary tract, etc.). Surgery is efficient, but pain relief is sometimes incomplete, and recurrence of pain is observed in seven to 28 per cent of patients within two years of the surgery. Around 20 per cent of patients undergo further surgery in the following two and a half years, indicating the recurrence of the endometriotic lesion(s). Unlike medical treatment, surgery may provide benefits for fertility. However, endometrioma surgery can negatively impact ovarian reserve and does not improve the outcome of in vitro fertilisation (IVF) in infertile patients.
The fertility treatment (Kalaitzopoulos et al., 2021; Singh et al., 2020) mostly relies on IVF. Endometriosis is known to contribute to subfertility via several mechanisms: pelvic adhesions, distorted pelvic anatomy, bilateral tubal blockage, poor quality of the oocyte/embryo and/or endometrial environment. Interestingly, it was shown that deferring the embryo transfer in endometriosis-affected women was associated with significantly higher cumulative pregnancy rates (Bourdon et al., 2018), probably due to a better endometrial environment in a cycle without ovarian stimulation. Concerning the response to IVF fertility treatment, a meta-analysis (Barbosa et al., 2014) showed that women with endometriosis have practically the same chance of achieving clinical pregnancy and live birth as women with other causes of infertility. IVF success rate is around 40 per cent (with a strong influence being the patient’s age at the time of egg retrieval).
So, treatment options are imperfect and very limited data are available to predict which treatment will work for which patient.
Endometriosis pathophysiology is not well understood
The strongest hypothesis for the origin of endometriotic lesions is retrograde menstruation, a reflux of menstrual blood through the fallopian tubes into the peritoneal cavity. But as this phenomenon occurs in 90 per cent of menstruating women and only 1 in 10 develops endometriosis, other mechanisms are at play. Individual susceptibilities involving intrinsic specific alterations of eutopic (uterine) and/or ectopic endometrium (high survival capacity, invasive and proliferative capabilities, somatic mutation), as well as alterations of the local peritoneal environment (excessive inflammation, defective immune clearance of ectopic cells, oxidative stress) are thought to be key aspects of the pathophysiology.
Indeed, endometriotic lesions have an altered gene expression profile compared to eutopic endometrium (normally localised within the uterus) (Borghese et al., 2008), with increased survival and adhesion signatures. The eutopic endometrium from endometriosis patients is also altered compared to healthy control, with notable differences involved in immunity (Rai et al., 2010; Poli-Neto et al., 2020). A single-cell transcriptomic study on endometrium from healthy donors, endometrium and endometriotic lesion from endometriosis patients (all obtained by invasive procedures) showed that several cell subtypes from the patients’ eutopic endometrium share alterations with endometriotic lesions (Ma et al., 2021). An aberrant immune response seems crucial for enabling the ectopic proliferation of endometrial cells (Vallvé-Juanico, Houshdaran and Giudice, 2019). Not only do peritoneal immune cells fail to clear the endometriotic lesions, but they also appear to promote their proliferation and invasion capabilities (Jeung, Cheon, and Kim, 2016; Chan et al., 2017). Immunomodulation may be an interesting strategy for the treatment of endometriosis (Jeljeli et al., 2020). As immune cells are involved in implantation and placentation (Ander, Diamond, and Coyne, 2019), modulating them may benefit fertility. The MultiMENDo project’s coordinator led a study showing that pre-conceptional immunomodulation alters immune cell recruitment at the maternal-foetal interface in mice (Dang et al., 2021). In the inflammatory context of endometriosis, such an effect may be beneficial. Menstrual immune cells are also refluxed in the peritoneal cavity, but they were scarcely studied in endometriosis (Schmitz et al., 2021; Warren et al., 2018), and their potential pathogenic role remains to be evaluated.
Menstrual blood is an easily accessible yet understudied biological fluid
Menstrual blood is an easily accessible biological fluid available monthly in non-pregnant women of reproductive age. Amongst the naturally available biological fluids, it is by far one of the least studied, with 37 to 189 times fewer biomedical research studies for menstrual blood than other biological fluids (peripheral blood, saliva, urine samples, faeces/stool, or seminal fluid/sperm). This illustrates that menstrual blood has been greatly overlooked as a biological fluid so far.
The increased popularity of reusable items of personal feminine hygiene products, such as the menstrual cup, makes it very easy to collect. This collection method greatly improves the possibility of studying viable cells (immune and endometrial cells) from this fluid and secreted factors in the menstrual serum. Menstrual immune cells resemble the uterine microenvironment more than the circulating immune cells (van der Molen et al., 2014). While menstrual blood is easily accessible and relevant to both gynaecological disorders and fertility, there is an extremely low number of studies on this biological fluid. Most studies focused on its use as a source of mesenchymal stem cells for regenerative therapies (Lv et al., 2018). A few studies using menstrual blood in the context of endometriosis have shown some promise (Nikoo et al., 2014; Warren et al., 2018; Schmitz et al., 2021; Shih et al., 2022), but so far, the number of included patients was limited. Collecting both menstrual immune and endometrial cells from a higher number of women with no or different types of endometriosis is therefore relevant for a search for endometriosis biomarkers that could truly impact diagnosis, understanding and treatment of this disease.
MultiMENDo project
The MultiMENDo project aims to identify relevant differences in menstrual blood between healthy donors and those with endometriosis. The project also looks at variations within subgroups of people with endometriosis. These differences could become reliable signs for diagnosing or predicting outcomes (biomarkers). MultiMENDo seeks to understand how endometriosis develops and explore new ways to treat it.
For this, single-cell transcriptomics and soluble protein multiplex assays will be used on 64 menstrual blood samples to identify candidate diagnostic biomarkers that differentiate endometriosis-affected women from healthy controls. Validation of these biomarkers will be carried out in menstrual blood samples from 250 women (200 endometriosis-affected women with different subtypes of endometriosis). Prognostic candidate biomarkers for response to surgery and in vitro fertilisation will be identified in menstrual blood from patients with longitudinal monitoring. Menstrual-fluid-derived organoids cultured with or without immune cells will be used to assess endometriosis-associated functional changes and to test new immunomodulatory treatments. The MultiMENDo project will lead to better endometriosis care and improve our understanding of endometriosis pathophysiology. It will also broaden the study of menstrual blood, a greatly overlooked biological fluid relevant to gynaecological and reproductive disorders.
References
Ander, S.E., Diamond, M.S. and Coyne, C.B. (2019) ‘Immune responses at the maternal-fetal interface’, Science Immunology, 4(31), p. eaat6114. doi: 10.1126/sciimmunol.aat6114.
Barbara, G., Buggio, L., Facchin, F. and Vercellini, P. ‘Medical Treatment for Endometriosis: Tolerability, Quality of Life and Adherence’, Frontiers in Global Women’s Health, 2, p. 729601. doi: 10.3389/fgwh.2021.729601.
Barbosa, M.A.P., Teixeira, D.M., Navarro, P.A.A.S., Ferriani, R.A., Nastri, C.O. and Martins, W.P. (2014) ‘Impact of endometriosis and its staging on assisted reproduction outcome: systematic review and meta-analysis’, Ultrasound in Obstetrics & Gynecology, 44(3), pp. 261–278. doi: 10.1002/uog.13366.
Barra, F., Grandi, G., Tantari, M., Scala, C., Facchinetti, F. and Ferrero, S. (2019) ‘A comprehensive review of hormonal and biological therapies for endometriosis: latest developments’, Expert Opinion on Biological Therapy, 19(4), pp. 343–360. doi: 10.1080/14712598.2019.1581761.
Becker, C.M., Gattrell, W.T., Gude, K. and Singh, S.S. (2017) ‘Reevaluating response and failure of medical treatment of endometriosis: a systematic review’, Fertility and Sterility, 108(1), pp. 125–136. doi: 10.1016/j.fertnstert.2017.05.004.
Borghese, B., Mondon, F., Noël, J.-C., Fayt, I., Mignot, T.-M., Vaiman, D. and Chapron, C. (2008) ‘Research Resource: Gene Expression Profile for Ectopic Versus Eutopic Endometrium Provides New Insights into Endometriosis Oncogenic Potential’, Molecular Endocrinology, 22(11), pp. 2557–2562. doi: 10.1210/me.2008-0322.
Bourdon, M. Santulli, P., Maignien, C., Gayet, V. Pocate-Cheriet, K., Marcelling, L. and Chapron, C. (2018) ‘The deferred embryo transfer strategy improves cumulative pregnancy rates in endometriosis-related infertility: A retrospective matched cohort study’, PloS One, 13(4), p. e0194800. doi: 10.1371/journal.pone.0194800.
Brulport, A., Bourdon, M., Vaiman, D., Drouet, C. Pocate-Cheriet, K., Bouzid, K., Marcellin, L., Santulli, P., Abo, C., Jeljeli, M., Chouzenoux, S., Chapron, C., Batteux, F., Berthelot, C. and Doridot, L. (2024) ‘An integrated multi-tissue approach for endometriosis candidate biomarkers: a systematic review’, Reproductive Biology and Endocrinology, 22(1), p. 21. doi: 10.1186/s12958-023-01181-8.
Chan, R.W.S., Lee, C.-L., Ng, E.H.Y. and Yeung, W.S.B. (2017) ‘Co-culture with macrophages enhances the clonogenic and invasion activity of endometriotic stromal cells’, Cell Proliferation, 50(3). doi: 10.1111/cpr.12330.
Chapron, C., Marcelling, L., Borghese, B. and Santulli, P. (2019) ‘Rethinking mechanisms, diagnosis and management of endometriosis’, Nature Reviews. Endocrinology, 15(11), pp. 666–682. doi: 10.1038/s41574-019-0245-z.
Dang, Y., Souchet, C., Moresi, F., Jeljeli, M., Raquillet, B., Nicco, C., Chouzenoux, S., Lagoutte, I., Marcellin, L., Batteux, F. and Doridot, L. (2021) ‘BCG-trained innate immunity leads to fetal growth restriction by altering immune cell profile in the mouse developing placenta’, Journal of Leukocyte Biology [Preprint]. doi: 10.1002/JLB.4A0720-458RR.
Donnez, J. and Dolmans, M.-M. (2021) ‘Endometriosis and Medical Therapy: From Progestogens to Progesterone Resistance to GnRH Antagonists: A Review’, Journal of Clinical Medicine, 10(5), p. 1085. doi: 10.3390/jcm10051085.
Ghai, V., Jan, H., Shakir, F., Haines, P. and Kent, A. (2020) ‘Diagnostic delay for superficial and deep endometriosis in the United Kingdom’, Journal of Obstetrics and Gynaecology, 40(1), pp. 83–89. doi: 10.1080/01443615.2019.1603217.
Gupta, D., Hull, M.L., Fraser, I., Miller, L., Bossuyt, P.M.M., Johnson, N. and Nisenblat, V. (2016) ‘Endometrial biomarkers for the non-invasive diagnosis of endometriosis’, The Cochrane Database of Systematic Reviews, 4, p. CD012165. doi: h10.1002/14651858.CD012165.
Jeljeli, M., Riccio, L.G.C, Chouzenoux, S., Moresi, F., Toullec, L., Doridot, L., Nicco, C., Boudon, M., Marcellin, L., Santulli, P., Abrão, M.S., Chapron, C. and Batteux, F. (2020) ‘Macrophage Immune Memory Controls Endometriosis in Mice and Humans’, Cell Reports, 33(5), p. 108325. doi: 10.1016/j.celrep.2020.108325.
Jeung, I., Cheon, K. and Kim, M.-R. (2016) ‘Decreased Cytotoxicity of Peripheral and Peritoneal Natural Killer Cell in Endometriosis’, BioMed Research International, 2016, p. 2916070. doi: 10.1155/2016/2916070.
Kalaitzopoulos, D.R., Samartzis, N., Kolovos, G.N.,Mareti, E., Samartzis, E.P., Eberhard, M., Dinas, K. and Daniilidis, A. (2021) ‘Treatment of endometriosis: a review with comparison of 8 guidelines’, BMC Women’s Health, 21(1), p. 397. doi: 10.1186/s12905-021-01545-5.
Leone Roberti Maggiore, U., Ferrero, S., Mangili, G., Bergamini, A., Inversetti, A., Giorgione, V., Viganò, P. and Candiani, M. (2016) ‘A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes’, Human Reproduction Update, 22(1), pp. 70–103. doi: 10.1093/humupd/dmv045.
Lv, H., Hu, Y., Cui, Z. and Jia, H. (2018) ‘Human menstrual blood: a renewable and sustainable source of stem cells for regenerative medicine’, Stem Cell Research & Therapy, 9(1), p. 325. doi: 10.1186/s13287-018-1067-y.
Ma, J., Zhang, L., Zhan, H., Mo, Y., Ren, Z., Shawo, A. and Lin, J. (2021) ‘Single-cell transcriptomic analysis of endometriosis provides insights into fibroblast fates and immune cell heterogeneity’, Cell & Bioscience, 11(1), p. 125. doi: 10.1186/s13578-021-00637-x.
Macer, M.L. and Taylor, H.S. (2012) ‘Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility’, Obstetrics and Gynecology Clinics of North America, 39(4), pp. 535–549. doi: 10.1016/j.ogc.2012.10.002.
van der Molen, R.G., Schutten, J.H.F., van Cranenbroek, B., ter Meer, M., Donckers, J., Scholten, R.R. can der Heijden, O.W.H., Spaanderman, M.E.A. and Joosten, I. (2014) ‘Menstrual blood closely resembles the uterine immune micro-environment and is clearly distinct from peripheral blood’, Human Reproduction, 29(2), pp. 303–314. doi: 10.1093/humrep/det398.
Nikoo, S., Ebtekar, M., Jeddi-Tehrani, M., Shervin, A., Bozorgmehr, M., Vafaei, S., Kazemnejad, S. and Zarnari A.-H. (2014) ‘Menstrual blood-derived stromal stem cells from women with and without endometriosis reveal different phenotypic and functional characteristics.’, Molecular Human Reproduction, 20(9), pp 905–918. doi: 10.1093/molehr/gau044.
Nisenblat, V., Bossuyt, P.M.M., Shaikh, R., Farquhar, C., Jordan, V., Scheffers, C.S., Mol, B.W.J., Johnson, N. and Hull, M.L. (2016a) ‘Blood biomarkers for the non-invasive diagnosis of endometriosis’, The Cochrane Database of Systematic Reviews, (5), p. CD012179. doi: 10.1002/14651858.CD012179.
Nisenblat, V., Prentice, L., Bossuyt, P.M.M., Farquhar, C., Hull, M.L. and Johnson, N. (2016b) ‘Combination of the non-invasive tests for the diagnosis of endometriosis’, The Cochrane Database of Systematic Reviews, 7, p. CD012281. doi: 10.1002/14651858.CD012281.
Poli-Neto, O.B., Meola, J., Rosa-e-Silva, J.C. and Tiezzi, D. (2020) ‘Transcriptome meta-analysis reveals differences of immune profile between eutopic endometrium from stage I-II and III-IV endometriosis independently of hormonal milieu’, Scientific Reports, 10(1), p. 313. doi: 10.1038/s41598-019-57207-y.
Rai, P., Kota, V., Deendayal, M and Shivaji, S. (2010) ‘Differential proteome profiling of eutopic endometrium from women with endometriosis to understand etiology of endometriosis’, Journal of Proteome Research, 9(9), pp. 4407–4419. doi: 10.1021/pr100657s.
Schmitz, T., Hoffman, V., Olliges, E., Bobinger, A., Popovici, R., Nößner, E. and Meissner, K. (2021) ‘Reduced frequency of perforin-positive CD8+ T cells in menstrual effluent of endometriosis patients’, Journal of Reproductive Immunology, 148, p. 103424. doi: 10.1016/j.jri.2021.103424.
Shih, A.J., Adelson, R.P., Vashistha, H., Khalili, H., Nayyar, A., Puran, R., Herrera, R., Chatterjee, P.K., Lee, A.T., Truskinovsky, A.M., Elmaliki, K., DeFranco, M., Metz, C.N. and Gregersen, P.K. (2022) ‘Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis’, BMC Medicine, 20(1), p. 315. doi: 10.1186/s12916-022-02500-3.
Simoens, S., Dunselman, G., Dirksen, C., Hummelshoj, L., Bokor, A., Brandes, I., Brodszky, V., Canis, M., Colombo, G.L., DeLeire, T., Falcone, T., Graham, B., Halis, G., Horne, A., Kanj, O., Kjer, J.J., Kristensen, J., Lebovic, D., Mueller, M., Viganò, P., Wullschleger, M. and D’Hooghe, T. (2012) ‘The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres’, Human Reproduction, 27(5), pp. 1292–1299. doi: 10.1093/humrep/des073.
Singh, S.S., Gude, K., Perdeaux, E., Gattrell, W.T. and Becker, C.M. (2020) ‘Surgical Outcomes in Patients With Endometriosis: A Systematic Review’, Journal of Obstetrics and Gynaecology Canada, 42(7), pp. 881–888.e11. doi: 10.1016/j.jogc.2019.08.004.
Vallvé-Juanico, J., Houshdaran, S. and Giudice, L.C. (2019) ‘The endometrial immune environment of women with endometriosis’, Human Reproduction Update, 25(5), pp. 564–591. doi: 10.1093/humupd/dmz018.
Warren, L.A., Shih, A., Renteira, S.M., Seckin, T., Blau, B., Simpfendorfer, K., Lee, A., Metz, C.N. and Gregersen, P.K. (2018) ‘Analysis of menstrual effluent: diagnostic potential for endometriosis.’, Molecular Medicine, 24(1). doi: 10.1186/s10020-018-0009-6.
de Ziegler, D., Pirtea, P., Carbonnel, M., Poulain, M., Cicinelli, E., Bulletti, C., Kostaras, K., Kontopoulos, G., Keefe, D., Ayoubi, J.M. (2019) ‘Assisted reproduction in endometriosis’, Best Practice & Research Clinical Endocrinology & Metabolism, 33(1), pp. 47–59. doi: 10.1016/j.beem.2018.10.001.
Project name
MultiMENDo project
Project summary
The MultiMENDo project focuses on endometriosis, a gynaecological disorder affecting approximately 10 per cent of women of childbearing age. This complex disease is notably associated with chronic pelvic pain and infertility, leading to a reduced quality of life. There is a huge diagnostic delay and a lack of curative therapies. The project aims to find diagnostic and prognostic biomarkers and investigate new therapeutic approaches using menstrual blood, a relevant and easily accessible yet overlooked biological fluid.
Project lead profile
Ludivine Doridot is a researcher at INSERM (French National Institute of Health and Medical Research) and an Associate Professor at Université Paris Cité (Paris, France). She obtained her PhD in Genetics from Université Paris Descartes in 2013 for her studies on preeclampsia, a hypertensive disease of pregnancy. She then performed a postdoc in Beth Israel Deaconess Medical Center, a Harvard-affiliated hospital in Boston (USA), where she studied genetic-environment interaction in the context of metabolic syndrome. Since 2017, she has focused on endometriosis and reproductive immunology.
Project contacts
Ludivine Doridot
Batiment Gustave Roussy, 4ème étage
22 rue Mechain, 75014 Paris, France
LinkedIn: /ludivine-doridot/
Funding
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101078556.
Figure legends
Figure 1: Endometriosis is characterised by lesions of ectopic endometrium. Schematic representation of the healthy (A) and endometriosis-affected (B) female reproductive tract with its surroundings. The positioning of a menstrual cup is shown (A). Lesion site of abnormally located endometrium (ectopic) defines the three commonly used subtypes (superficial, ovarian and deep infiltrating). The uterine endometrium changes during the menstrual cycle, and it is shed during the menses (C). Source: BioRender.com.