Revelation after three decades of ablation therapy: two loops instead of a single re-entry loop drive atrial tachycardia
Nele Vandersickel, Sander Hendrickx, Robin Van Den Abeele
Atrial tachycardia: driven by a single re-entry circuit?
Normal heart contractions begin with an electrical impulse originating at the sinoatrial node in the right atrium. This impulse spreads throughout both atria and then proceeds via the atrioventricular (AV) node to the remainder of the heart. Atrial tachycardia (AT) is a common arrhythmia affecting these atria (about 1/10 000 people have this arrhythmia). It is frequently attributed to an anatomical re-entry pathway disrupting the heart’s natural rhythm. Anatomical re-entry occurs when the electrical impulse circulates around an obstacle, such as a valve, vein or scar tissue, resembling the motion of a Mexican wave. This self-sustaining rotation is typically rapid and supersedes the heart’s normal rhythm governed by the sinoatrial node.
The prevailing consensus, in cases where AT is induced by re-entry, is that it is usually driven by a single re-entry circuit. AT can be effectively managed with catheter ablation, a well-established medical procedure with a history spanning over three decades (Ghzally, Ahmed and Gerasimon, 2023). In this procedure, a specialised catheter is introduced through a percutaneous access point, often located in the femoral or groin region. Within the heart chambers, the catheter functions as a precise tool for meticulously mapping the activation pattern of the targeted atrium. Additionally, entrainment mapping, a technique involving pacing manoeuvres, can help identify the primary re-entry circuit. Once the re-entry pathway is identified, it can be interrupted by creating controlled, minute lesions or scars blocking its passage, like building a wall in the middle of your Mexican wave.
Nonetheless, despite the interruption of the re-entry circuit, the AT often persists. Our analysis of approximately 150 cases in our database revealed that this phenomenon occurs in approximately one-third of the cases. Additionally, our review of the existing literature yielded consistent findings (Arantes et al., 2011; Takigawa et al., 2019; Kaiser, Rogers and Narayan, 2019). The observation that AT often transforms into a slower rhythm following ablation suggests the possibility of a secondary loop that plays a crucial role in ablation therapy. Interestingly, this is currently completely missed by activation mapping and entrainment mapping. What is particularly intriguing is the limited research conducted to understand the underlying reasons for this phenomenon (Takigawa et al., 2019).
We embarked on a comprehensive investigation of this phenomenon with the support of my ERC Starting Grant. We found some very interesting and unexpected results. We found that single loops do not exist and that the prevailing consensus is wrong. Moreover, an old theorem, over 30 years old, published in old physics journals, proved that single loops are impossible to exist on a closed surface. The left or right atrium can be considered closed surfaces with holes. This has far-reaching consequences on the current view of the mechanism of the AT and how it should be ablated. Let us explain in more detail in the next sections.
Looking at the heart as a topological object
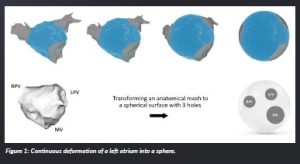
In simple terms, topology is about understanding certain properties of objects and spaces that do not change, even if you stretch or squish them, as long as you do not make new openings or close existing ones. When we look at the left and right atrium in the heart, they each have three natural openings or holes. In the left atrium are the mitral valve, the left pulmonary veins and the right pulmonary veins. In the right atrium are three openings: the tricuspid valve, the inferior vena cava and the superior vena cava. In topological terms, this means we can reshape both the left atrium and the right atrium into a sphere with these three holes without changing their fundamental properties. However, when we create lines during a medical procedure like ablation to connect two of these holes, it essentially reduces them to a single hole in terms of topology.
Computer simulation revealed the second loop
Within this simplified spherical model with holes, we conducted over 1 200 simulations to investigate re-entrant patterns in AT. We initiated re-entry by creating a scenario where electrical impulses circulated around one of these holes. Subsequently, we virtually intervened by connecting any two of these holes, effectively ’ablating’ the re-entrant circuit. Notably, the choice of which holes to connect directly impacted the results. In some instances, the AT terminated; in others, a new AT emerged, often with a slower activation rate.
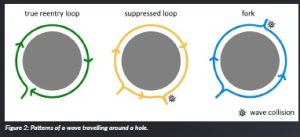
Upon closer examination of the newly established re-entrant circuit in cases where the AT was slower, we discerned an intriguing phenomenon. Prior to ablation, this particular re-entrant loop appeared quiescent—completing nearly a full rotation before being abruptly halted. This arrest in rotation occurred because the dominant and evidently driving re-entrant loop (we will call this the type true loops) collided with the other loop, effectively suppressing it. We will call this type of loop suppressed loops.
Our research comprised over 1 200 simulations, encompassing diverse settings, consistently yielded the same outcomes. Notably, in scenarios involving three holes, we consistently observed the presence of two true re-entrant loops. These two loops could jointly drive the AT, implying that neglecting to ablate one of the loops would lead to the generation of a new AT with the same activation rate. However, in some cases, a true re-entrant loop coexisted with a secondary suppressed loop. In such instances, the failure to ablate the suppressed loop resulted in a new AT with a slower activation rate.
Our findings challenge the prevailing notion of single re-entrant loops in AT. Instead, our simulations consistently revealed their existence in pairs. The reason behind the oversight of this second loop is attributed to its subtle presence on conventional activation maps. Consequently, this suppressed loop was overlooked in the entire history of ablation therapy as not being important.
A very old theorem confirms our findings
Remarkably, our findings align with an established theorem dating back to 1988, known as the index theorem, which was articulated in studies by Winfree and Davidsen (Winfree and Tyson, 1988; Davidsen, Glass and Kapral, 2004). This theorem postulates a fundamental principle: on a closed surface, the total count of clockwise re-entries should precisely match the total count of counterclockwise re-entries. Astonishingly, our simulations corroborated this theorem’s assertion.
However, a crucial revelation emerged during our investigation: we recognised that a suppressed loop also qualifies as a re-entry within the context of the index theorem. Astonishingly, despite the theorem’s existence for decades, its connection to the underlying mechanism of ATs remained unexplored and unacknowledged throughout this time.
Profound implications on ablation therapy
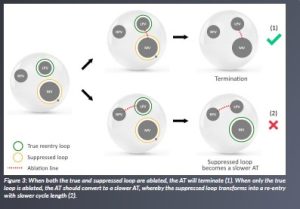
Our discoveries carry profound implications for the field of ablation therapy. Neglecting to ablate the second loop emerges as the pivotal factor contributing to the onset of a slower AT.
Remarkably, in instances where the initial ablation line successfully halted the AT, it was serendipitously due to the simultaneous ablation of both loops. This outcome occurred even when electrophysiologists identified only a single loop.
However, armed with our newfound understanding, we recognise the necessity of ablating both loops within the common channel, as visually depicted in Figure 3. This critical insight marks a transformative shift in the approach to ablation therapy for AT, holding the potential to significantly enhance its effectiveness. We have over 35 cases that all confirm our theory.
Software package DGM-TOP can automatically find all loops
There is a notable absence of software tools capable of automatically identifying both loops during ablation procedures. Existing mapping software primarily trains cardiac electrophysiologists (EPs) to pinpoint single re-entry circuits. However, we have pioneered the development of a proprietary software package, DGM- TOP, that can discern and track both loops throughout ablation therapy.
While our innovative software, DGM-TOP, has yet to be fully integrated into clinical practice, we hold the aspiration that mapping companies will recognise its potential and adopt it for widespread use. To facilitate this integration, we remain committed to substantiating our findings and showcasing DGM- TOP’s efficacy in identifying both loops, whether they are true or suppressed, particularly in complex cases.
In instances where patients exhibit scar tissue within the atria, additional anatomical complexities arise, creating new ‘holes’ around which true or suppressed loops may form. Consequently, we are diligently working to expand the capabilities of DGM-TOP to detect scar tissue and analyse the associated patterns automatically.
To comprehensively validate our software and introduce this scar tissue detection feature, we are initiating a collaborative study involving 15 hospitals in Belgium with the help of an ERC proof-of-concept grant. This collective effort aims to further assess and enhance the functionality of DGM-TOP, ultimately advancing the field of ablation therapy.
What lies ahead?
We express our profound gratitude for the invaluable support extended to us through the ERC Starting Grant. Our journey has taken us far beyond the boundaries of our original project scope.
Remarkably, we never anticipated that the field of topology would unveil the essential insights needed to understand ATs comprehensively.
Our achievement has been made possible by seamlessly integrating diverse disciplines, including mathematics, physics (computer simulation), software development and clinical electrophysiology. This interdisciplinary synergy has been instrumental in our ability to unlock this previously uncharted territory. It underscores the notion that thinking beyond the confines of one’s own field often paves the way for the discovery of truly unexpected and groundbreaking results.
Our curiosity is not limited to the impact of our findings on ATs alone; we are equally eager to explore how our results may extend to other, even more critical and life-threatening arrhythmias. The potential implications for broader arrhythmia research are indeed tantalising. The fundamental principles and insights we have uncovered may serve as a stepping stone for understanding and addressing a wide spectrum of arrhythmias that pose greater risks to patients’ lives. Our commitment to pushing the boundaries of arrhythmia research remains steadfast as we aspire to contribute to the advancement of knowledge and treatments for these more severe and complex cardiac conditions.
References
Arantes, L., Klein, G. J., Jaïs, P., Lim, K. T., Matsuo, S., Knecht, S., Hocini, M., O’Neill, M. D., Clémenty, J. and Haïssaguerre, M. (2011) ‘Tachycardia transition during ablation of persistent atrial fibrillation’, Journal of Cardiovascular Electrophysiology, 22(5), pp. 506–512. doi: 10.1111/j.1540-8167.2010.01964.x.
Davidsen, J., Glass, L. and Kapral, R. (2004) ‘Topological constraints on spiral wave dynamics in spherical geometries with inhomogeneous excitability’, Physical Review E, 70(5), 056203. doi: 10.1103/PhysRevE.70.056203.
Ghzally, Y., Ahmed, I. and Gerasimon, G. (2023) ‘Catheter Ablation’, in: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK470203/.
Kaiser, D. W., Rogers, A. J. and Narayan, S. M. (2019) ‘Predictability in complex atrial arrhythmias: The N/N-1 algorithm to guide ablation of atrial tachycardias’, Heart Rhythm, 16(4), pp. 562–563. doi: 10.1016/j.hrthm.2018.11.018.
Takigawa, M., Derval, N., Martin, C. A., Vlachos, K., Denis, A., Kitamura, T., Cheniti, G., Bourier, F., Lam, A., Martin, R., Frontera, A., Thompson, N., Massoullié, G., Wolf, M., Duchateau, J., Klotz, N., Pambrun, T., Sacher, F., Cochet, H., Hocini, M., Haïssaguerre, M. and Jaïs, P. (2019) ‘A simple mechanism underlying the behavior of re-entrant atrial tachycardia during ablation’, Heart Rhythm, 16(4), pp. 553–561. doi: 10.1016/j.hrthm.2018.10.031.
Winfree, A. T. and Tyson, J. J. (1988) ‘When Time Breaks Down: The Three‐Dimensional Dynamics of Electrochemical Waves and Cardiac Arrhythmias’, Physics Today, 41(12), 107–109. doi: 10.1063/1.2811674.
Project profile
Project name
SMARTHEART
Project summary
There is an urgent need to better understand and localise the sources of cardiac arrhythmia in order to improve its treatment. SmartHeart proposes a radical new approach—applying network theory to study cardiac arrhythmia mechanisms. Network theory has myriad applications throughout biology, physics and social sciences. However, it has never been applied to the heart. In this proposal, we will apply network theory to clinical data of cardiac arrhythmia, backed up by computer simulations, to automatically detect the source of the arrhythmia for complex atrial tachycardia, atrial fibrillation and ventricular tachycardia. As we will identify possible ablation targets, the goal of this project is to improve the treatment for the patient.
Project lead profile
Nele Vandersickel is accociate professor at Ghent University. She obtained her PhD in high energy physics in 2011 and switched her research field to biophysics when starting a post-doc. She has experience in analysing experimental data, clinical data and performing computer modelling and aims to merge these three different components in her work during this grant to improve the health of the patient.
Project contacts
Nele Vandersickel
Krijgslaan 281, S9 9000 Ghent, Belgium.
0032 497 15 34 36
www.dgm.eu (under construction)
Funding
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. DLV-900008.
Image legends
Figure 1: Continuous deformation of a left atrium into a sphere.
Figure 2: Patterns of a wave travelling around a hole.
Figure 3: When both the true and suppressed loop are ablated, the AT will terminate (1). When only the true loop is ablated, the AT should convert to a slower AT, whereby the suppressed loop transforms into a re-entry with slower cycle length (2).