Dr Christos Beregeles and Dr Daniel Leff
on behalf of the MAMMOBOT consortium
A new clinical application for surgical robotics could prove more accurate than a mammogram.
Over 55 000 breast cancers are diagnosed every year in the UK alone (Cancer Research UK, n.d.). Early diagnosis of breast cancer is critical to increasing patients’ chances of survival. Breast screening programmes using mammograms can help detect breast cancer before the patient experiences any symptoms. However, many ‘early’ breast cancers still require surgery, radiotherapy and chemotherapy. Some early changes in breast tissue have to be examined under a microscope to confirm whether they are cancerous; this results in patients having to undergo invasive surgical excision or vacuum biopsy to remove large tissue volumes when the vast majority will not have the disease (Rudin et al., 2017). Similarly, women presenting with single duct nipple discharge (SDND), in whom only 3-6 per cent will harbour malignancy (Dillon et al., 2006), undergo blind exploratory surgical intervention.
Arguably, there needs to be a re-framing of what constitutes ‘early’ breast cancer, with platforms for detecting and excluding the disease far earlier to increase survival and reduce morbidity associated with current treatments. As many types of breast cancer start within the cells that line a breast duct, it is illogical to wait for external signs of breast cancer to develop (e.g. mass, micro-calcifications) and diagnose the disease from the outside. Approximately 80–90 per cent of breast cancers are intra-ductal (American Cancer Society, n.d.) (in the lining of a breast duct). A framework that enables navigation, interrogation, and intervention within the ductal network (mammary ductoscopy) could completely disrupt how breast cancer is diagnosed and treated. The nipple areolar is a natural orifice that can theoretically facilitate such mammary duct tree evaluation.
Mammary ductoscopy has shown tremendous promise in diagnosing and excluding breast cancer in patients with SDND (Bender et al., 2009; Liu et al., 2008; Filipe et al., 2015; Waaijer et al., 2016). Duct wall irregularities, epithelial thickening, and inflammatory changes can all be visualised (Waaijer et al., 2016). Despite its potential, the technique has not been widely adopted in the UK because current platforms are unwieldy, inflexible, and cannot safely, swiftly and smoothly navigate down the complex tree-like structure of the mammary ducts.
‘Growing robots’
Growing robots (Hawkes et al., 2017; Greer et al., 2019) can ‘unfold’ inside the mammary ducts via the addition of material at their tip and therefore minimise disruption of the anatomy. Robotic catheters, on the other hand, rely on insertion through pushing, implying harmful frictional forces between the catheter and duct. This safety characteristic of growing robots is well suited to mammary ductoscopy as it allows movement within the breast ducts (1-8mm) with minimal collateral damage. Growing robots also have the intrinsic property of having a hollow core that can be used as an instrument channel or guide.
MAMMOBOT
The MAMMOBOT project comprises three overarching objectives:
- build a flexible miniature robot that can safely fit inside the breast duct lumen
- navigate the growing robot through the complex duct tree structure
- provide the robot with enhanced sensing capabilities to characterise luminal tissues and provide decision support.
The MAMMOBOT proof-of-concept is a flexible growing ductoscopic robot created for early breast cancer detection and has already been developed. One of the first millimetre-scale steerable soft growing robots, MAMMOBOT is designed to enter the breast via the nipple and then navigate the mammary ducts to detect abnormal cells that could be a pre-cursor to invasive breast cancer.
MAMMOBOT comprises a growing robot pressurised with a saline solution and a robotic catheter responsible for steering. Saline solution is used, as opposed to air, as it is preferred in clinical applications to prevent the risk of air embolism. The saline solution also acts as a lubricant as it fills the entire growing element and prevents dry friction during eversion.
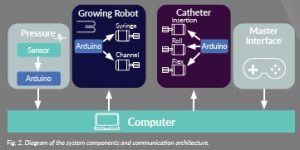
The developed growing robot architecture allows for an active inner instrument channel that can be used to pass the robotic catheter, a camera, or other types of instruments such as biopsy needles, snares, or fibreoptic sensors for in situ histopathology. The robotic catheter is tendon driven and is inserted through the inner channel, enabling both growing/retraction and robot steering in any direction. The system architecture is depicted in Figure 1.
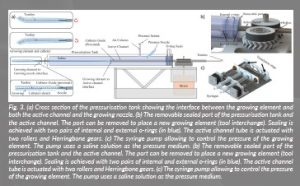
The growing element is connected to the nozzle of the pressurisation tank on one end (see ‘growing element to growing nozzle interface’ in Figure 3a) and to the active inner channel tube on the other end (see ‘growing element to active channel interface’ in Figure 3a). It is made of a hollow tube made from 35 μm-thick LDPE (low density polyethylene) film. The tube is everted around itself longitudinally at the tip as in typical existing growing robot systems (Figure 3a).
The robot control concept works as follows: pressure is increased to a set value which induces robot growth. Subsequently, the active channel is pushed forward together with the catheter. Simultaneous actuation of the catheter’s roll and bending angles allows the direction of robot growth to be controlled. The stiffness and the shape of the distal catheter, as well as the pressure inside the growing element, have an impact on the overall steering capabilities of the robot (Berthet-Rayne et al., 2021).
Progress: navigation within a breast phantom
MAMMOBOT has partially navigated a slightly scaled-up (x 1.5) realistic mammary duct phantom. A life-cast of a female adult’s breast, a 3D model of the internal breast structures, and 2D anatomical drawings were used as
a reference.
The ductal tree branch was created by sculpting a magnified version of the 3D printed branch used for reference. The ducts of the branch are of 2–3 mm diameter and each ends into a hollow lobule. The branch was created by a composition of a network of tubes simulating the ‘negative space’ of the internal structure of the ductal tree. Then the sculpted branch was used as a mould to create the ductal branch with shore 2A platinum silicone. Once the ductal tree was cured, it was pinned onto position on the breast and connected with silicone.
The robot could fit within the ductal tree and be successfully removed, as shown in Figure 3.
Future work will investigate alternative materials for the growing element to improve pressure resistance and decrease internal friction, theoretical investigation of growing sheath buckling, and realistic environment interaction. Following the observation that fluid pressure can be the predominant factor in determining the overall system stiffness, modifications of the modelling approach will be considered. Finally, a miniature endoscopic camera will allow exploration of the anatomy with improved precision, opening doors to autonomous navigation.
Impact
Presently, there is no minimally invasive robotic platform for the exploration of the mammary ducts for early detection and treatment of atypical ductal proliferations and/or non-invasive disease. MAMMOBOT is a novel platform that can progress in situ diagnosis and treatment in breast cancer. The potential translational impact of MAMMOBOT is enormous: as a supplementary screening test to reduce the one missed cancer per 2 500 women screened with mammography (NHS, n.d.), to curtail 0.68-1.22 interval cancers per 1 000 women screened (Bennett, Sellars, and Moss, 2011; Dibden, 2014), as a platform to better ‘map out’ diseased ducts to improve the precision of conventional resection (Woodward, 2010) and finally, over the long-term, as a potential platform for delivery of novel targeted and ablative therapies (Mauri et al., 2017).
A public and patient involvement (PPI) committee will be created as part of the MAMMOBOT project, including patients having routine mammographic screening via the NHS, high-risk screening due to genetic mutations, and who have undergone microdochetomy for SDND. The group will specifically advise on population acceptance, targeting and frequency of evaluation and potential risks. We will also canvass wider patient views by presenting our proposal to patients at the Maggies Centre (Charing Cross Hospital), which runs a ‘science engagement café’. Furthermore, we will leverage local and national academic advice and support for the project through our involvement in the CRUK ICR-ICL major centre (research sub-committee) and the Association of Breast Surgery (academic research committee).
In the longer term, by securing additional future grants, we hope to continue developing MAMMOBOT to meet regulatory approval ready for first in women studies.
References
American Cancer Society (no date) Invasive Breast Cancer (IDC/ILC). Available at: https://www.cancer.org/ cancer/breast-cancer/about/types-of-breast-cancer/invasive-breast-cancer.html.
Bender, O., Balci, F.L., Yüney, E. and Akbulut, H. (2009) ‘Scarless endoscopic papillomectomy of the breast’, Onkologie, 32(3), pp. 94–98. doi: 10.1159/000195694.
Bennett, R.L., Sellars, S.J. and Moss, S.M. (2011) ‘Interval cancers in the NHS breast cancer screening programme in England, Wales and Northern Ireland’, British Journal of Cancer, 104(4), pp. 571–577. doi: 10.1038/bjc.2011.3.
Berthet-Rayne, P., Hadi Sadati, S.M., Petrou, G., Patel, N., Giannarou, S., Leff, D.R., and Bergeles; C. (2021) ‘MAMMOBOT: A Miniature Steerable Soft Growing Robot for Early Breast Cancer Detection’, IEEE Robotics and Automation Letters, 6(3), pp. 5056–5063. doi: 10.1109/LRA.2021.3068676.
Cancer Research UK (no date) Breast Cancer Statistics. Available at: https://www.cancerresearchuk.org/ health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer.
Dibden, A., Offman, J., Parmar, D., Jenkins, J., Slater, J., Binysh, K., McSorley, J., Scorfield, S., Cumming, P., Liao, X-H., Ryan, M., Harker, D., Stevens, G., Rogers, N., Blanks, R., Sellars, S., Patnick, J. and Duffy, S.W.. (2014) ‘Reduction in interval cancer rates following the introduction of two-view mammography in the UK breast screening programme’, British Journal of Cancer, 110(3), pp. 560–564. doi: 10.1038/ bjc.2013.778.
Dillon, M.F., Mohd Nazri, S.R., Nasir, S. McDermott, E.W., Evoy, D., Crotty, T.B., O’Higgins, N. and Hill, A.D.K. (2006) ‘The role of major duct excision and microdochectomy in the detection of breast carcinoma’, BMC Cancer, 6, 164. doi: 10.1186/1471-2407-6-164.
Waaijer, L., van Diest, P.J., Verkooijen, H.M., Dijkstra, N-E., van der Pol, C.C., Borel Rinkes, I.H.M., Witkamp, A.J. (2015) ‘Interventional ductoscopy in patients with pathological nipple discharge’, British Journal of Surgery, 102(13), pp. 1639–1648. doi: 10.1002/bjs.9950.
Greer, J.D., Morimoto, T.K., Okamura, A.M. and Hawkes, E.W. (2019) ‘A Soft, Steerable Continuum Robot That Grows via Tip Extension’, Soft Robotics, 6(1), pp. 95–108. doi: 10.1089/soro.2018.0034.
Hawkes, E.W., Blumenschein, L.H., Greer, J.D. and Okamura, A.M. (2017) ‘A soft robot that navigates its environment through growth’, Science Robotics, 2(8), eaan3028. doi: 10.1126/scirobotics.aan3028.
Liu, G.Y., Lu, J.S., Shen, K.W., Wu, J., Chen, C.M., Hu, Z., Shen, Z.Z., Zhang, T.Q. and Shao, Z.M. (2008)
‘Fiberoptic ductoscopy combined with cytology testing in the patients of spontaneous nipple discharge’,
Breast Cancer Research and Treatment, 108(2), pp. 271–277. doi: 10.1007/s10549-007-9598-4.
Mauri, G., Sconfienza, L.M., Pescatori, L.C., Fedeli, M.P., Ali, M., Di Leo, G. and Sardanelli, F. (2017) ‘Technical success, technique efficacy and complications of minimally-invasive imaging-guided percutaneous ablation procedures of breast cancer: A systematic review and meta-analysis’, European Radiology, 27(8), pp. 3199–3210. doi: 10.1007/s00330-016-4668-9.
NHS (no date) How to decide if you want breast screening. Available at: https://www.nhs.uk/conditions/ breast-cancer-screening/why-its-offered/.
Rudin, A.V., Hoskin, T.L., Fahy, A., Farrell, A.M., Nassar, A., Ghosh, K. and Degnim, A.C. (2017) ‘Flat Epithelial Atypia on Core Biopsy and Upgrade to Cancer: a Systematic Review and Meta-Analysis’, Annals of Surgical Oncology, 24(12), pp. 3549–3558. doi: 10.1245/s10434-017-6059-0.
Waaijer, L., Simons, J.M., Borel Rinkes, I.H., van Diest, P.J., Verkooijen, H.M. and Witkamp, A.J. (2016) ‘Systematic review and meta-analysis of the diagnostic accuracy of ductoscopy in patients with pathological nipple discharge’, British Journal of Surgery, 103(6), pp. 632–643. doi: 10.1002/bjs.10125.
Woodward, S., Daly, C.P., Patterson, S.K., Joe, A.I. and Helvie, M.A. (2010) ‘Ensuring excision of intraductal lesions: marker placement at time of ductography’, Academic Radiology, 17(11), pp. 1444–1448. doi: 10.1016/j.acra.2010.06.014.
Figures
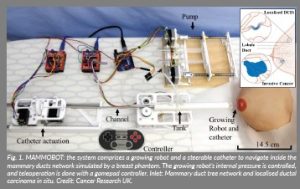
Figure 1: MAMMOBOT: the system comprises a growing robot and a steerable catheter to navigate inside the
mammary ducts network simulated by a breast phantom. The growing robot’s internal pressure is controlled,
and teleoperation is done with a gamepad controller. Inlet: Mammary duct tree network and localised ductal
carcinoma in situ. Credit: Cancer Research UK.
Figure 2: Diagram of the system components and communication architecture.
Figure 3: (a) Cross section of the pressurisation tank showing the interface between the growing element and
both the active channel and the growing nozzle. (b) The removable sealed port of the pressurisation tank and
the active channel. The port can be removed to place a new growing element (tool interchange). Sealing is
achieved with two pairs of internal and external o-rings (in blue). The active channel tube is actuated with
two rollers and Herringbone gears. (c) The syringe pump allowing to control the pressure of the growing
element. The pump uses a saline solution as the pressure medium. (b) The removable sealed port of the
pressurisation tank and the active channel. The port can be removed to place a new growing element (tool
interchange). Sealing is achieved with two pairs of internal and external o-rings (in blue). The active channel
tube is actuated with two rollers and Herringbone gears. (c) The syringe pump allowing to control the pressure
of the growing element. The pump uses a saline solution as the pressure medium.