Stimuli-responsive hybrid nanoconstructs for efficient theranostic applications in nanomedicine
Valentina Cauda, Silvia Appendino, Veronica Vighetto and Marco Carofiglio
Department of Applied Science and Technology, Politecnico di Torino, Italy
Cancer is the second most common cause of death worldwide; globally, one death out of six is due to cancer. Its incidence is constantly rising, and estimates say that by 2040 there will be 16.3 million yearly deaths due to cancer against the 10 million yearly deaths recorded in 2020 (WHO, 2022). The main drawbacks of traditional therapies are lack of selectivity, the insurgence of side effects and problems of recurrence and resistance. The research in the field of THERANOSTIC (thus THERApeutics and diagNOSTICS) nanomaterials is leading to a treatment approach customised for individual patients, with several aims: to develop efficient, targeted therapies, safe and efficacious; to eliminate the unnecessary treatment of patients; to localise the site of disease or the disease state; to monitor the response to the treatment; to obtain a better molecular understanding of cancer biology; to obtain significant cost savings for the overall healthcare system.
In this context, the recently concluded ERC Starting Grant project TrojaNanoHorse (TNH) and the following ERC Proof-of-Concept project XtraUS, both led by Prof. Valentina Cauda, developed a hybrid biomimetic nanoconstruct for cancer imaging and treatment aiming to cover the gap between the present nanomedicine tools and the clinical requirements.
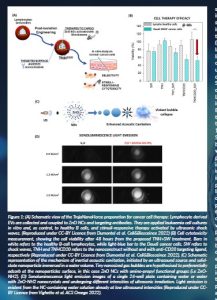
The TNH nanoconstruct was developed as a core-shell nanoparticle possessing a stimuli-responsive core made of zinc oxide nanocrystals and an unconventional biomimetic shell formed by a lipid bilayer derived from extracellular vesicles (EVs) and targeting ligands to specifically catch cancer cells (Figure 1A). The ZnO core was selected due to its manifold properties, such as the capability to be therapeutically activated by a mechanical pressure wave, like ultrasound (Vighetto et al., 2019; Racca et al., 2020), and to thus produce damage and toxic species on-demand when internalised in cancer cells. Furthermore, this nanocrystalline ZnO core can be used as a bioimaging nanocontrast agent once acoustically activated, making it an interesting theranostic tool (Ancona et al., 2020; Vighetto et al., 2022). The ZnO nanocrystalline core was synthesised by a wet-chemical solvothermal synthesis assisted by microwave, leading to highly reproducible ZnO nanocrystals in terms of size distribution, morphology and surface chemistry (Garino et al., 2019; Cauda et al., 2017). However, to further increase the nanocrystals’ biocompatibility and low cellular toxicity, together with outstanding and long-term colloidal stability in ethanol and water media, further optimisation was achieved by synthesising iron-doped ZnO nanocrystals (Carofiglio et al., 2021a). These doped nanocrystals have the further advantage of magnetic responsiveness, which is very useful to make nanocrystals work as contrast imaging agents in MRI for future clinical perspectives.
The shell of the TNH nanoconstruct was a nature-mimicking lipid bilayer shell produced from EVs. The team proceeded to the isolation and full characterisation of these biovesicles derived from different cell lines, particularly from cancer and healthy cells cultured in vitro. The biological role of lymphocyte-derived EVs was also studied in terms of intrinsic homing capability towards their healthy parental cell (B lymphocytes) and towards cancer cells, both lymphoid (Daudi) and myeloid (HL60) cell lines. Data showed the high affinity between the native lymphocyte-derived EVs and the Daudi cancer cell line, underlining that this type of EV is significantly internalised more by their healthy parental cells and by their cancer counterpart Daudi than by HL60. We also proposed a further re-engineering of EVs, bioconjugating them with the monoclonal antibody anti-CD20 (Rituximab) in order to have a specific cancer cell targeting (Limongi et al., 2021). We highlighted indeed that EVs bioconjugated with antiCD20 have a specific targeting exclusively to the CD20+ Daudi cancer cell line and not towards B lymphocytes or the CD20- HL60 cancer cell.
The surface modification of naturally-produced EVs with a specific antibody is part of a broader re-engineering approach to EVs, which can be accompanied by the modification of the EV’s cargo (Susa et al., 2019; Villata et al., 2020). By preparing our TNH nanoconstruct, we obtained fully nanotechnology-re-engineered EV, loaded with an active theranostic cargo (ZnO NCs) and enriched at the surface with ligands for successful cancer cell targeting.
The ZnO nanocrystalline core was actively loaded by means of a temporary permeabilisation of EVs membrane through a freeze-thaw method, obtaining a high-yield encapsulation. Furthermore, the antiCD20 antibody was bioconjugated to the EV surface, creating the final TNHCD20 nanoconstruct. The monodispersed size distribution, the colloidal stability over long time periods of the TNH, its haemocompatibility and biocompatibility were then all successfully assessed; the targeting selectivity of the TNHCD20 towards Daudi cancer cells, sparing healthy B lymphocytes (also CD20+) and cancer HL60 cells (which are CD20-negative cells) was also efficaciously demonstrated (Dumontel, 2022). With the TNHCD20 weapon prepared against leukaemia cancer cells, we then studied the therapeutic and bioimaging capabilities. The TNHCD20 showed a stimuli-responsive ability under different mechanical pressure waves in both cases. Depending on the stimulation, we could achieve a therapeutic activity, without using drugs, directed against cancer cells and sparing the healthy ones, or echogenic and sonoluminescent signals which can be efficiently used for imaging, having the TNH or even the sole ZnO NCs as nanocontrast agents.
Concerning the therapeutic activity, the synergism between the TNHCD20 and the applied shock waves (SWs), a specific type of mechanical pressure waves, was proven to kill Daudi cancer cells and spare healthy B lymphocytes, in vitro (Dumontel et al., 2022) (Figure 1B).
As preliminarily observed (Racca et al., 2020), the best irradiation parameters with SW should be screened to obtain the desired cytotoxic effects in synergy with sole ZnO NCs. In particular, SWs were administered by the high-energy focalised SW device (PiezoWave2 from R. Wolf, ELvation Medical), with an intensity of 12.5 MPa, a number of 250 used shots, distributed in three SW applications every 3h per day. The SWs alone caused a slight decrease in the cell proliferation in adenocarcinoma KB cell line, while the combination with pure ZnO NCs showed an important cytotoxicity. The same result was then reported with the final TNHCD20 nanocostruct, showing remarkable and selective toxicity with SWs directed to leukaemic cancer cells with respect to the healthy lymphocytes (Dumontel, 2022). The observed cytotoxicity could be caused by the combination of various concurrent effects leading to the mechanical injury of the cell structure: (i) an enhanced acoustic bubble cavitation; (ii) the ‘nanoscalpel effect’ supported by the preferred interaction of the TNHCD20 construct with the Daudi cell line; and (iii) an electric charge imbalance, due to the piezoelectric behaviour of ZnO nanostructures (Carofiglio et al., 2021b).
Despite the fact that the biomolecular mechanism of the induced cell death is still being studied, it is noteworthy that the observed enhanced cytotoxicity is due to the concomitant administration of the SWs and of the targeting TNHCD20, thus proving that the TNH hybrid nanoconstruct developed within the project can be activated on-demand by external stimulation, such as acoustic waves, efficiently achieving a high cytotoxic effect against targeted cancer cells while being highly biocompatible towards healthy cells.
Concerning the imaging capability of the TNH nanoconstruct, the sonoluminescence (SL) has been investigated in water and biological solutions using the ZnO nanocrystals as novel nanocontrast agents. SL is acknowledged as the emission of light resulting from the implosion of cavitating bubbles that form in a liquid when irradiated by US (Figure 1C). As described in Vighetto (2021) and Cauda et al. (2021), an experimental setup for sonoluminescence spectroscopy and imaging was implemented. To perform sonication, the sample well was placed in contact with a commercial ultrasonic transducer with a planar geometry (LipoZero G39, Globus) through a thin layer of ecographic coupling gel. The light emission produced from the well containing the sample was acquired using a multicore optical UV–vis fibre connected to a monochromator (Acton SP 2300); the spectral signal was collected by a CCD camera (Princeton LN) operating at −90 °C. The ultrasound irradiation power was varied from 20 to 80 per cent of the maximum power of the ultrasonic transducer, i.e. from 0.6 to 2.4 W/cm2, duty cycle (DC) equal to 100 per cent, and 1 MHz of frequency. SL spectra were measured from the beginning of sonication, and images were also recorded. To explore the potential applications of SL for biological imaging, several spectra were obtained in different biologically relevant media irradiated both with and without pure ZnO NCs, as preliminary data. Irrespective of the medium used, the cutoff in the UV range produced by the presence of semiconducting NCs was evident. It is thus possible to confirm the capability to have an SL emission from various water-based media and that the presence of ZnO NCs can impart a clear signature to the I spectrum. Moreover, by comparing the acquired images at different US power intensities, it is evident how the SL light emitted in the presence of ZnO NCs is higher than that in their absence. Figure 1D clearly shows the threshold of SL at 0.9 W/cm2 of US power, where sonoluminescence is actually visible only for the nanocrystal water dispersion.
The measurements evidenced the increase of SL emission, as the ZnO NCs concentration in aqueous solutions increases, and the decrease of the US power necessary to detect the SL signal when NCs are present. It was indeed inferred that the NP surface is able to trap nanosized gas bubbles (Ancona et al., 2020; Vighetto et al., 2022), which are responsible for the enhanced cavitation and, thus, for efficient SL imaging (Figure 1C).
These results clearly indicate an SL generation enhancement by ZnO NCs, confirming that the developed TNH can also be successfully exploited as a novel nanocontrast agent.
Conclusion
In conclusion, thanks to the TrojaNanoHorse ERC Starting Grant, not only has the synthesis of the TNH nanoconstruct been successfully achieved, but it has also been demonstrated that it has all features to become a powerful theranostic biomimetic nanodevice.
A project follow-up was then achieved in the ERC Proof-of-Concept Grant XtraUS, where the targeted TNH was further applied to colorectal cancer cells to induce metastasis. Indeed, this project aims to fight circulating tumour cells (CTCs) from colorectal cancer, which circulate in the bloodstream, in order to fight them before the establishment of the metastasis. The obtained data show that the TNH or simple ZnO NCs combined with multiple SW treatments allow a dramatic proliferation loss in colorectal cancer cells of two different types (HT-29 and colo320DM). These promising data are under study in 3D models and in vivo mouse xenografts to prove the efficacy of the proposed therapy and its future clinical translation.
References
Ancona, A., Troia, A., Garino, N., Dumontel, B., Cauda, V. and Canavese G. (2020) ‘Leveraging Re-Chargeable Nanobubbles on Amine-Functionalized ZnO Nanocrystals for Sustained Ultrasound Cavitation towards Echographic Imaging’, Ultrasonics Sonochemistry, 67, 105132. doi: 10.1016/j.ultsonch.2020.105132.
Carofiglio, M. Laurenti, M., Vighetto, V., Racca, L., Barui, S., Garino, N., Gerbaldo, F., Laviano, F., Cauda, V. (2021a) ‘Iron-Doped ZnO Nanoparticles as Multifunctional Nanoplatforms for Theranostics’, Nanomaterials, 11, pp. 2628. doi: 10.3390/nano11102628.
Carofiglio, M., Laurenti, M., Graziana Genchi, G., Ciofani, G., Grochowicz, M. and Cauda, V. (2021b) ‘Ultrasound Triggered ZnO-Based Devices for Tunable and Multifaceted Biomedical Applications’, Advanced Materials Interfaces, 2101021, pp. 1–14. doi: 10.1002/admi.202101021.
Cauda, V., Canavese, G., Limongi, T., Garino, N., Laurenti, M., Racca, L., Ancona, A., Canta, M. and Dumontel, B. (2017) Biomimetic Non-Immunogenic Nanoassembly for the Antitumor Therapy. World Intellectual Property Organization no. WO2019/092550. Available at: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2019092550.
Cauda, V., Vighetto, V., Carofilgio, M. and Ancona, A. (2021) A sonoluminescence contrast imaging method and apparatus assisted by nanoparticles in the biomedical field, in particular in the oncological field. Italian patent application N. IT102021000005123.
Dumontel, B., Susa, F., Limongi, T., Vighetto, V., Debellis, D., Canta, M. and Cauda, V. (2022) ‘Nanotechnological Engineering of Extracellular Vesicles for the Development of Actively Targeted Hybrid Nanodevices’, Cell & Bioscience 12(61), pp. 1–18. doi: 10.1186/s13578-022-00784-9.
Garino, N., Limongi, T., Dumontel, B., Canta, M., Racca, L., Laurenti, M., Castellino, M., Casu, A., Falqui, A. and Cauda, V. (2019) ‘A Microwave-Assisted Synthesis of Zinc Oxide Nanocrystals Finely Tuned for Biological Applications’, Nanomaterials, 9 (212), pp. 1–19. doi: 10.3390/nano9020212.
Limongi, T., Susa, F., Dumontel, B., Racca, L., Perrone Donnorso, M., Debellis, D. and Cauda V. (2021) ‘Extracellular Vesicles Tropism: Innate Passive and Active Engineered Targeting Capability Comparison’, Membranes, 11, pp. 886. doi: 10.3390/membranes11110886.
Racca, L., Limongi, T., Vighetto, V., Dumontel, B., Ancona, A., Canta, M., Canavese, G., Garino, N. and Cauda, V. (2020) ‘Zinc Oxide Nanocrystals and High-energy Shock Waves: a New Synergy for the Treatment of Cancer Cells’, Frontiers in Bioengineering and Biotechnology – Nanobiotechnology, 8, pp. 577. doi: 10.3389/fbioe.2020.00577.
Susa, F., Limongi, T., Dumontel, B., Vighetto, V. and Cauda, V. (2019) ‘Engineered Extracellular Vesicles as a Reliable Tool in Cancer Nanomedicine’, Cancers 11(12), pp. 1979. doi: 10.3390/cancers11121979.
Vighetto, V., Ancona, A., Racca, L., Limongi, T., Troia, A., Canavese, G. and Cauda, V. (2019) ‘The Synergistic Effect of Nanocrystals Combined with Ultrasound in the Generation of Reactive Oxygen Species for Biomedical Applications’, Frontiers in Bioengineering & Biotechnology – Nanobiotechnology, 7, pp. 374. doi: 10.3389/fbioe.2019.00374.
Vighetto, V., Troia, A., Laurenti, M., Carofiglio, M., Marcucci, N., Canavese, G. and Cauda, V. (2022) ‘Insight into Sonoluminescence Augmented by ZnO-Functionalized Nanoparticles’, ACS Omega, 7(8), pp. 6591–6600. doi: 10.1021/acsomega.1c05837.
Villata, S., Canta, M. and Cauda, V. (2020) ‘EVs and Bioengineering: From Cellular Products to Engineered Nanomachines’, International Journal of Molecular Sciences, 21(17), pp. 6048. doi: 10.3390/ijms21176048.
WHO (2022) Cancer. Available at: https://www.who.int/news-room/fact-sheets/detail/cancer (Accessed: 2 September 2022).
Project summary
The multidisciplinary ERC Starting Grant project “Hybrid immune-eluding nanocrystals as smart and active theranostic weapons against cancer” (TrojaNanoHorse) and the following ERC Proof-of-Concept Grant aim to develop a new generation of multifunctional theranostic nanosystems and apply them for improved cancer treatment, efficient cell imaging and for providing high safety for the hosting organism.
Project lead profile
Prof. Valentina Cauda is a full professor of nanotechnology and leads a research group of 20 people at Politecnico di Torino, Turin (Italy). After receiving her ERC Starting Grant in 2015, she founded the TrojaNanoHorse Laboratory, in brief TNHLab, to create novel hybrid and biomimicking nanomaterials. Prof. Cauda has authored over 130 peer-reviewed articles and seven international patents.
Project partner
The TrojaNanoHorse project is based at Politecnico di Torino (Italy), taking advantage of its state-of-the-art nanomaterial laboratories and knowledge. The main research topics are theranostic nanomaterials, extracellular vesicles and their re-engineering for advanced drug delivery and nanoimaging vehicles. Collaborations include groups from the University of Turin, other EU countries (Germany, the Netherlands, Spain) and non-EU countries (the USA, Chile and Australia).
Contact details
Prof. Valentina Cauda
Corso Duca degli Abruzzi 24,
10129 Torino, Italy
+39 011 090 7389
FUNDING
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreements No. 678151 and No. 957563.
Figure legends
Figure 1: (A) Schematic view of the TrojaNanoHorse preparation for cancer cell therapy: Lymphocyte derived
EVs are collected and coupled to ZnO NCs and targeting antibodies. They are applied leukaemia cell cultures
in vitro and, as control, to healthy B cells, and stimuli-responsive therapy activated by ultrasonic shock
waves. (Reproduced under CC-BY Licence from Dumontel et al. Cell&Biosceince 2022). (B) Cell cytotoxicity
measurement, showing the cell viability after 48 hours from the proposed TNH+SW treatment. Bars in
white refers to the healthy B-cell lymphocytes, while light-blue bar to the Daudi cancer cells. SW refers to
shock waves, TNH and TNHCD20 refers to the nanoconstruct without and with anti-CD20 targeting ligand,
respectively (Reproduced under CC-BY Licence from Dumontel et al. Cell&Biosceince 2022). (C) Schematic
representation of the mechanism of inertial acoustic cavitation, initiated by an ultrasound source and solidstate
nanoparticle immersed in a water volume. Tiny nanosized gas bubbles are hypothesised to preferentially
adsorb at the nanoparticles surface, in this case ZnO NCs with amino-propyl functional groups (i.e ZnONH2).
(D) Sonoluminescence light emission images of a single 24-well plate containing water or water
with ZnO-NH2 nanocrystals and undergoing different intensities of ultrasonic irradiation. Light emission is
evident from the NC-containing water solution already at low ultrasound intensities (Reproduced under CCBY
Licence from Vighetto et al. ACS Omega 2022).