Dr Sarah Guerin, University of Limerick
Researchers from the University of Limerick in Ireland have developed a way to crystallise amino acid molecules, the building blocks of proteins found in our body, into discs and plates for use as sensors. These amino acid crystals are piezoelectric, meaning that they can convert mechanical energy into electricity.
For the first time, these crystals can be made into any shape, significantly enhancing their potential for applications in medical devices and infrastructure damage detection (Hari et al., 2024). When tapped, these discs and plates generate voltage, which could be amplified to charge electronic devices using everyday movements.
This research, funded by the European Research Council (ERC), aims to replace current piezoelectric materials that contain harmful elements like lead.
Piezoelectric materials are improving the efficiency and eco-friendliness of many current and emerging technologies in medical device, infrastructure, automotive and aerospace industries. As they can generate electricity from ambient mechanical stimuli, they facilitate self-powered electronic devices, reducing reliance on batteries. Unfortunately, most commercial piezoelectric components contain lead zirconium titanate (PZT) made up of 60 wt% lead oxide (PbO). Despite the massive environmental and health impact of PZT, its unparalleled performance has allowed it to remain a stark exception to EU and global regulations. Prominent alternative lead-free piezoelectrics rely on elements such as barium, bismuth and niobium, which also carry onerous and irreversible environmental costs.
Our ERC Starting Grant project Pb-FREE (Piezoelectric biomolecules for lead-free, Reliable, Eco-Friendly Electronics) is systematically designing biomolecular crystal device components based on simple biomolecules. Our goal is to advance these materials to outperform and phase out PZT and its competitors to provide cost-effective, eco-friendly, technology-ready piezoelectric materials. By rapidly accelerating the path through design, growth and engineering, Pb-FREE is establishing biomolecular crystals as a new class of high-performance, sustainable and eco-friendly piezoelectric materials.
What needs to be done
As suggested by their multitude of applications, piezoelectric materials and their corresponding device components come in an almost endless variety of shapes, sizes, hardness, rigidity and chemical composition. Many state-of-the-art piezoelectric materials are geared towards flexible sensing, which has distinctly different requirements and applications versus ceramic-based device components. This is similar to avenues such as energy harvesting and in vivo applications that are more accessible areas of exploration for biological piezoelectrics, including amino acid and peptide crystals, fibrous proteins and organic polymers.
The Pb-FREE programme is focused on replacing the most ubiquitous and more challenging piezoelectric components that are currently fabricated using hard, rigid, perovskite-based ceramics with large piezoelectric responses. These are used as actuators in medical devices and vehicles, as well as motors, printers, scientific equipment, sonar navigation and much more.
All ceramics, as well as piezoelectric polymers such as polyvinylidene fluoride (PVDF), require a process known as poling (application of a strong electric field) to induce a piezoelectric response. This high-temperature process is just one of up to 12 steps in ceramic fabrication, including high-temperature sintering and calcification, further increasing the economic and environmental price of processing these materials.
Small biomolecular crystals are unique in that they can form rigid macroscopic assemblies. If moulded, standardised and insulated, these assemblies can easily replace inorganic ceramic-based device components. Crystalline amino acids can be quickly and easily grown at room temperature with no by-products and do not require poling, as is necessary for PZT, sodium potassium niobate (KNN), barium titanate (BaTiO3) and other ceramics.
A brief history of biological piezoelectricity
Many biological materials naturally lack a centre of symmetry in their crystal structure, endowing them with piezoelectric properties. In the past 70 years, piezoelectricity has been confirmed in a variety of biological materials, including wood and bone, and in fibrous proteins like collagen, chitin and elastin, which present as highly ordered crystalline molecules in mammalian tissue.
Until the twenty-first century, biological piezoelectricity was not considered technologically significant, with experimental values ranging from 0.1–2 pC/N. The piezoelectric response of amino acid crystals was first discussed and qualified by Lemanov, and a small number of single amino acid crystals began to be synthesised and characterised.
The giant piezoelectric response and ferroelectricity found in glycine stimulated intensive research aimed at controlled growth of glycine polymorphs and at creating biocompatible piezoelectric sensors more broadly to work in direct contact with a biological environment. In 2017, we discovered giant piezoelectricity in a form of glycine. This discovery also included the quantification of significant piezoelectricity in most protein-forming amino acids in single crystal form, based on computational predictions.
Since then, amino acid nanocrystals have been grown in alumina pores for the detection of ultra-low mechanical pressures and have been deposited via electrospinning, highlighting their sensitivity and versatility. Peptide crystals continue to be developed as energy harvesters that are grown on rigid silicon substrates and packaged for electrical measurements.
Strength in numbers: polycrystalline assemblies
While high-quality, large single crystal growth is preferable in applications such as non-linear optics, this process can take weeks or months for biomolecular crystals. It requires very controlled temperatures that significantly increase energy consumption and, thus, the economic and environmental costs of fabrication. Even then, very few biomolecular single crystals can achieve the millimetre scale necessary for the precise cutting required in many piezoelectric technologies, especially compared to industry mainstays such as quartz (SiO2), potassium dihydrogen phosphate (KDP) and lithium niobate (LiNbO3 ).
Polycrystalline films provide a unique, eco-friendly, cost-effective means of introducing biomolecular materials into mainstream technologies. Polycrystalline amino acid films can be grown quickly in ambient conditions and display versatile properties and growth mechanisms more akin to piezoelectrics like aluminium nitride and zinc oxide.
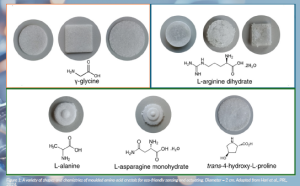
One of the main focuses of the project is the development of a standardised growth process for a polycrystalline biomolecular device component. Key to this is the recent validation of a new moulding technique for polycrystalline deposition, where layers of polycrystalline amino acids are deposited into, for example, disc-shaped silicon moulds to build a cohesive macroscopic structure. The resulting piezoelectric component structure has been optimised so that a standard piezoelectric element can be fabricated for a given solution concentration and volume and left to evaporate for a fixed amount of time. The entire growth process is carried out at room temperature with no by-products. We have shown that polycrystalline films can be grown in a variety of shapes and thicknesses via a novel moulded evaporation technique carried out for the first time in this project (Bhattacharya et al., 2024; Hari et al., 2024). The fabrication process has only five steps currently: weighing, mixing, a series of cycles of deposition and evaporation, followed by extraction from silicon moulds, and polishing.
Achievement unlocked: case studies in moulded biomolecular crystals
Piezoelectric and ferroelectric technologies are currently dominated by perovskite-based ceramics due to their impressive figures of merit and their versatility in size and shape. This allows their dimensions to be tailored to the needs of thousands of applications across the aforementioned industries. In this project, we are significantly advancing the performance and customisation of biomolecular crystal assemblies by growing them as moulded, substrate-free piezoelectric elements. This methodology allows for electromechanical properties to be embedded in these assemblies by fine-tuning the chemistry of the biomolecules and, thus, the functional properties of the single crystal space group. Our recent 2024 papers report multiple uses of this versatile, low-cost, low-temperature growth method that opens the path to phase-in biomolecular piezoelectrics as high-performance, eco-friendly alternatives to ceramics.
To date, in this field, free-standing, flexible PVDF nanocomposite films have been formed by drop casting the solution onto glass plate followed by thermal annealing and peeling off from the substrate. Likewise, peelable β-glycine/chitosan films and CBZ-Phe/ PDMS films have been made. Now, we have grown biomolecular crystals as stand-alone macroscopic piezoelectric elements without relying on substrates or polymers. While much of the research in this field has focused on polymer-centred flexible amino acid materials, these moulded amino acid assemblies are the first to potentially challenge the wide variety of thick, rigid and dimensionally diverse ceramic-based piezoelectric device components.
What has been missing to date in the effort to accelerate the uptake of biomolecular crystals as high-performance commercial piezoelectrics is a technique for growing polycrystalline assemblies in a repeatable and versatile manner at the macro-scale. Before this research, biomolecular piezoelectric materials were fabricated as single crystals, as thin films made via self-assembly on conductive substrates, e.g. Cu or membrane or polymer-based flexible materials. This technique allows us to have full control over the dimensions of the material, which allows it to be easily incorporated into various electromechanical applications.
As a part of our research to exploit amino acids as alternative piezoelectric materials, three challenges persisted:
- Forming single crystals and polycrystalline thin films of amino acids is challenging with molecules such as lysine.
- The piezoelectric responses of polycrystalline films are consistently lower than in single crystal form, sometimes by an order of magnitude.
- Amino acid crystals tend towards having high shear piezoelectricity, but little to no longitudinal piezoelectricity.
This summer, we reported a multicomponent crystal composed of mandelic acid and the amino acid lysine, which has overcome all these challenges. The multicomponent crystal has a naturally occurring longitudinal piezoelectric response, meaning the electricity is generated along the same axis as the applied force. The self-assembly of these crystals into a polycrystalline disc increases the piezoelectric response as opposed to dampening it, and the cocrystallisation forms stable crystals containing lysine, which has been predicted to have a high piezoelectric response. While the influence of shear contributions in piezoelectrics has to date existed only as a frustration when trying to measure isolated responses at the micro and nanoscale, this methodology allows for shear responses to be harvested and exploited without the need for complex sample geometries or bendable cantilever structures.
These exciting developments allow us to move towards the next phases of the project—screening the diverse chemistries of biomolecular crystals for high piezoelectricity to grow using this new technique and developing standardised procedures for insulating and electroding these eco-friendly piezoelectric discs and plates.
References
Bhattacharya, S., Cazade, P.-A., Hari, K., Ryan, T., Keeney, L., O’Mahony, C. and Guerin, S. (2024) ‘Harvesting of shear piezoelectricity in a molded multicomponent crystal disc’, Applied Materials Today, 39, 102344. doi: 10.1016/j.apmt.2024.102344.
Bishara, H., Nagel, A., Levanon, M. and Berger, S. (2020) ‘Amino acids nanocrystals for piezoelectric detection of ultra-low mechanical pressure’, Materials Science and Engineering: C, 108, 110468. doi: 10.1016/j.msec.2019.110468.
Chen, Y., Guerin, S., Yuan, H., O’Donnell, J., Xue, B., Cazade, P.A., Haq, E.U., Shimon, L.J.W., Rencus-Lazar, S., Tofail, S.A.M., Cao, Y., Thompson, D., Yang, R. and Gazit, E. (2022) ‘Guest molecule-mediated energy harvesting in a conformationally sensitive peptide-metal organic framework’, Journal of the American Chemical Society, 144(8), pp. 3468–3476. doi: 10.1021/jacs.1c11750.
Chorsi, M.T., Le, T.T., Lin, F., Vinikoor, T., Das, R., Stevens, J.F., Mundrane, C., Park, J., Tran, K.T.M., Liu, Y., Pfund, J., Thompson, R., He, W., Jain, M., Morales-Acosta, M.D., Bilal, O.R., Kazerounian, K., Ilies, H. and Nguyen, T.D. (2023) ‘Highly piezoelectric, biodegradable, and flexible amino acid nanofibers for medical applications’, Science Advances, 9(24), eadg6075. doi: 10.1126/sciadv.adg6075.
Fortunato, M., Chandraiahgari, C.R., De Bellis, G., Ballirano, P., Sarto, F., Tamburrano, A. and Sarto, M.S. (2018) ‘Piezoelectric effect and electroactive phase nucleation in self-standing films of unpoled PVDF nanocomposite films’, Nanomaterials, 8(9), pp. 1–20. doi: 10.3390/nano8090743.
Hari, K., Ryan, T., Bhattacharya, S. and Guerin, S. (2024) ‘Molded, solid-state biomolecular assemblies with programmable electromechanical properties’, Physical Review Letters. Accepted for publication. Available at: https://journals.aps.org/prl/accepted/2a079YcfD0c1c89b947f2d108c8258a5f9db4528a.
Hosseini, E.S., Manjakkal, L., Shakthivel, D. and Dahiya, R. (2020) ‘Glycine–chitosan-based flexible biodegradable piezoelectric pressure sensor’, ACS Applied Materials & Interfaces, 12(8), pp. 9008–9016. doi: 10.1021/acsami.9b21052.
Hu, T., Zhang, Z. and Reches, M. (2024) ‘A self-standing superhydrophobic material formed by the self-assembly of an individual amino acid’, Journal of Colloid and Interface Science, 655, pp. 899–908. doi: 10.1016/j.jcis.2023.11.062.
Lemanov, V.V. (2000) ‘Piezoelectric and pyroelectric properties of protein amino acids as basic materials of Soft State Physics’, Ferroelectrics, 238(1), pp. 211–218. doi: 10.1080/00150190008008786.
Project name
Pb-FREE
Project summary
Biomolecular crystals have emerged as exciting new sustainable piezoelectrics. Currently, little research is focused on developing these crystals as reliable, solid-state sensors to integrate into conventional electronic devices due to their water solubility, uncontrolled growth, variable response and electroding difficulties. Pb-FREE is taking on the challenge of accelerating the design, growth, and engineering of these novel piezoelectric materials through high-throughput computational screenings, novel crystal growth procedures, and standardised electromechanical characterisation and packaging.
Project lead profile
Dr Sarah Guerin runs the Actuate Lab in the Department of Chemical Sciences and Bernal Institute at the University of Limerick. She has secured over €2M of funding for her work on in-silico and ex-silico engineering of biomolecular crystals, primarily for eco-friendly sensing and pharmaceuticals. She works with research groups around the world to predict, understand and design the properties of functional molecular crystals. She graduated in 2015 with a BSc in Applied Physics from the University of Limerick, going on to complete her PhD in piezoelectric modelling. She is the 2023 SFI Early Career Researcher of the Year.
Project contacts
Dr Sarah Guerin
Bernal Institute, University of Limerick, Castletroy, Limerick City, Ireland.
Email: guerinactuatelab@gmail.com
Website: actuatelab.ie
X: @SPGuerin
LinkedIn: /sarah-guerin28/
Funding
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101039636.
Figure legends
Figure 1: A variety of shapes and chemistries of moulded amino acid crystals for eco-friendly sensing and actuating. Diameter = 2 cm. Adapted from Hari et al., PRL, 2024.